- Submissions

Full Text
Research & Development in Material Science
Design of Polyester Matrix Polymer Composites Reinforced with Jarosite Particulate Fillers: - Curing studies and Structural Durability Analysis
Muthusundar K
Peermohamed A
Ananthakumar S
1Materials Science and Technology Division CSIR-National Institute for Interdisciplinary Science and Technology, Thiruvananthapuram, India
Goutham N
2Digital University Kerala, India
Vaisakh SS
3Department of Chemistry, Sanatana Darma College, India
*Corresponding author:Ananthakumar S, Materials Science and Technology Division CSIR-National Institute for Interdisciplinary Science and Technology, Thiruvananthapuram, India
Submission: July 30, 2025;Published: September 03, 2025

ISSN: 2576-8840 Volume 22 Issue 2
Abstract
Industrial by-products especially inorganic oxides / silicates are cost effective particulate fillers preferred to develop low-cost, structural polymer composites and in turn promote the effective utilization of such by-products towards sustainable environment. In this work, Jarosite, chemically a ferro-silicate sulphate and a by-product produced by the zinc alloy processing industries, is reused as inorganic fillers for designing polyester polymer matrix structural composites. Cement is also blended with jarosite as a co-filler and stabilizer to make polyester composites. Curing studies of unsaturated polyester resin were first conducted with various percentage loading of jarosite and jarosite/cement blends by varying initiator concentrations. The workability of the polyester resin was systematically evaluated and optimized in terms of rheology, curing temperature and time. Composites were made by casting route and the end-products were assessed for the microstructure and mechanical properties such as compressive strength, mechanical wear in addition to water absorption and chemical stability. Results were correlated with the filler-matrix interaction with respect to filler loading. Polyester polymer matrix composites made with jarosite and cement reinforcements produce mechanically durable structural composites that can be used as alternative to the natural wood-based industrial products such as panels and boards needed in construction applications.
Keywords:Jarosite; Polyester resin; Polymer composites; Particulate filler; Curing; Tribology properties
Introduction
Development of particulate reinforced polymer matrix composites, mostly with oxides and silicates based inorganic nano/micro particulate fillers, impart high mechanical strength and structural durability to the matrix. Polymer matrix particulate composites found applications in fabricating ready-made cellular homes, roofing tiles and also composite panels/boards to substitute Natural wood [1-3]. Due to the technical and industrial importance of this subject, numerous studies have been conducted upon the effect of different kinds of particulate fillers in polymer matrix systems. Two critical factors have been reported; the interfacial adhesion between the filler particles and polymer matrix as well as the filler loading that decides the mechanical performance of the end-products. Moreover, the strength of a well-bonded particulate composite is accounted from the effective stress transfer from the matrix to the particles. Normally, the stiffness of the polymer matrix is improved with the addition of inorganic micro/nano phases having higher stiffness than the polymer matrix. Since polymer matrix exhibits poor surface properties, the demand for composites with high wear resistance is of great significance when extreme load bearing applications are considered. The wear properties are not entirely intrinsic material properties but are highly system dependent ones. In polymer matrix composites, it is expected that the geometrical factors like filler size, distribution of the filler material, preferred orientation and alignment relative to the wear surface and also their interaction with the composite matrix plays dominant role in determining the structural durability of the polymer matrix composites [4-10].
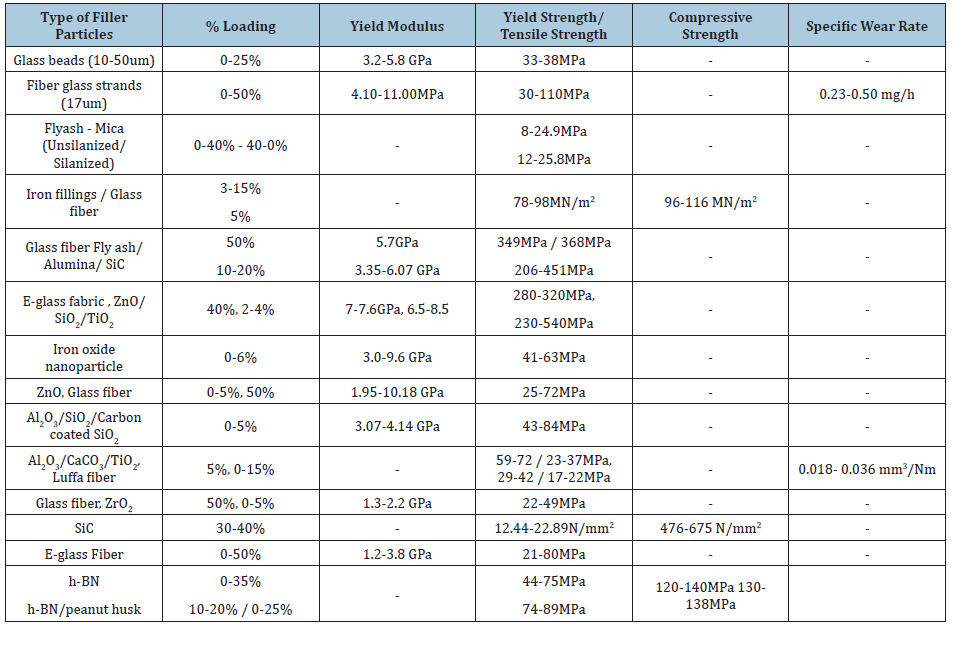
Unsaturated Polyester Resin (UPR) is a cost-effective thermosetting polymer industrially affordable for making composite structures. Even though the benzene rings and aliphatic segments of the polyester chain contribute to its stiffness, and good processability of the resin, the technical limitation realized in UPR is the brittleness after curing, due to their high level of cross-linking [11]. Therefore, it is highly necessary to understand the curing properties first with respect to the particulate reinforcements to limit the crack growth. Table 1 summarizes a few important reported Inorganic nano/micro fillers used in reinforcing polymer matrix with special reference to the Polyester polymer matrix [12- 25].
Table 1:Summary of the Inorganic fillers used for reinforcing polyester matrix along with mechanical properties reported in literature.
Recycling of inorganic industrial by-products set a new regime deemed for environmental, economic and social benefits. In this context, Jarosite used in this study is an industrial inorganic particulate material generated from the zinc alloy processing industries. Zinc alloys are obtained via hydro-metallurgy processing of ZnS ore. Jarosite is an industrial reject due to the presence of arsenic and lead impurities. However, utilization of jarosite in ceramic manufacturing by sintering technique is earlier reported [26-28]. Another eco-friendly approach is the fixing of Jarosite in polymer matrix for making structural composites. Published reports indicate no systematic work on Jarosite as reinforcing matter in any polymer matrix systems.
Jarosite is basically sodium or potassium ferrosilicate sulfate. It is usually treated with lime to make jarofix. Apart from lime, fixing jarosite in cement is another plausible route that has also not been reported earlier. The addition of a certain amount of ordinary Portland cement as a co-filler or stabilizer can neutralize the acidic jarosite and impart additional strength to the composite. XRF analysis shows that the chemical composition of the Portland cement contains nearly 69% calcium oxide and 25% of silicon dioxide. The analysis further confirmed the major mineral phase in cement are alite (69%) and beite (24%) [29]. Thus, the combined effect of lime as well as silica can be anticipated when jarosite and cement are used as reinforcements.
Therefore, in this study, jarosite as a particulate filler for making polyester polymer matrix composite is investigated for the first time. It is a novel idea for the effective immobilization and utilization of jarosite and also an alternate way for its’ safe disposal. In this paper, the effect of jarosite as the particulate filler, cement as co-filler is studied with polyester polymer matrix composites. Curing, water absorption, chemical stability mechanical strength, and tribological properties of the polyester composite are investigated and the results are discussed. The gel-time control of the composite melt is also explored to understand the working time of the melt. The effect of cement as the co-filler or additive in improving the bulk mechanical and tribological properties are also studied and documented.
Experimental
Materials
A commercial grade of thermosetting polyester resin, polyester resin GP-002, was purchased from the resin dealer Devi Chemicals, Thiruvananthapuram, [Kerala, INDIA] along with the catalyst, M.E.K.P (Methyl Ethyl Ketone Peroxide), and the accelerator, 6 % cobalt Naphthanate. The anhydrous Portland cement with brand name Sankar Cement, manufactured by the India Cements Limited, was procured from the local market. The jarosite used in the study was obtained from the company M/s Binani Zinc Limited, Kerala, and the chemical reagents that were used to create the alkaline (NaOH), acidic (HCl), saline (NaCl) mediums were supplied by the Fisher Scientific Ltd.
Fabrication of jarosite particle reinforced polyester matrix polymer composites
The as-received jarosite was dispersed mechanically in deionized water. The dispersion is then passed through the standard sieve [Mesh No: 350, 43 micron] and the fine micron sized jarosite particles are separated, dried and powdered for making composites. 100 parts of the polyester resin were weighed and warmed for about 15 minutes at a temperature of 55 ℃. The micron size jarosite powder was introduced into the low viscous polyester resin progressively. The mixture was slowly stirred along with the addition of the accelerator (10 parts). The stirring continued till the accelerator was homogeneously mixed. To this resin mix, measured quantity of initiator was added, stirred and finally transferred into a metallic mould kept at room temperature. The room temperature curing of the polyester composite was carefully monitored. The polyester-jarosite composites prepared with different weight percentages of jarosite were designated as PJ5, PJ10, PJ15, PJ20, PJ25, and PJ30 where P corresponds to polyester, J for jarosite, and 5, 10, 15, 20, 25 and 30 correspond to the weight % of jarosite particulates.
Fabrication of composites with cement as co-filler
The standard micron sized Portland cement and jarosite in 80:20 weight percentage was dry blended using a stationary blender. This jarosite-cement blend was progressively added to the weighed polyester resin (100 parts) maintained at 55 ℃. The accelerator (10parts) was added with this mixture and was stirred continuously until a homogeneous mixing was achieved. A measured amount of initiator was then added to this while stirring was continued for a while and finally was transferred to a metallic mould kept at room temperature. The curing was carried out at room temperature. The different polyester-jarosite-cement composites prepared with blended jarosite and cement were designated as PJC5, PJC10, PJC15, PJC20, PJC25, and PJC30 where P corresponds to polyester, J for jarosite, C for cement and 5, 10, 15, 20, 25 and 30 corresponds to the weight % of blended jarosite-cement particulate filler.
Characterizations
The particle size distribution and morphology of the jarosite powder as well as cement were studied using a Laser Diffraction Particle size analyzer (Beckman Coulter LS 13 320 particle size analyzer) and scanning electron microscope (JEOL 5600 SL, Japan) respectively. The chemical composition of jarosite and Portland cement powder was analyzed by the X-ray Fluorescence spectrometer (ED-XRF PANalytical Epsilon 3). The mineralogical characterization of the jarosite and Portland cement were carried out using the powder X-ray Diffractometer (Philips X’pertPro diffractometer) and were scanned in 2θ range of 10-90° using Cu Kα radiation (λ=1.54Å). The viscosity measurements of the neat polyester resin as well as the filler impregnated resin were carried out using a rotational viscometer (Anton Paar Rheo Viscometer) at room temperature with varying shear rates. The IR absorption of the fillers, matrix and the composites were analysed using Fourier Transform Infrared spectrometer (IR Prestige-21 FTIR-8400; Shimadzu Corporation, Japan) for the analysis of the filler-matrix interaction. The wear characteristics of the unsaturated polyesterjarosite (PJ) and unsaturated polyester-jarosite-cement (PJC) composites were also examined for better analysis of the surface morphology using the scanning electron microscope.
Determination of gel time and peak exothermic temperature
The curing of the composite melt was analyzed by the exotherm and gel time measurements as per the IS 13360 (Part 10/See 4): 2001. The resin mixed with the jarosite powder was treated with the initiator/catalyst and the accelerator as per the recommended ratio (1:5). The mixture was then poured into an aluminum foil dish kept at room temperature and was then allowed to cure. The gel time of the composite melted was then determined by measuring the resistance to the motion of an 8 mm diameter glass rod probe. In the meantime, the exotherm was measured by placing a thermocouple in the center of the catalyzed resin contained within the aluminum foil dish. The measurement of the gel time and the exotherm was then again studied by varying the initiator concentration by keeping the concentration of the accelerator constant.
Compressive strength
Compressive strength of the composites was analyzed by the aid of UNILAB Universal Testing Machine, at a constant cross head speed of 7mm min-1. The test specimens used for compression strength were prepared in cylindrical shape with 12mm diameterand 18mm height as per the standard ASTM D6641. The desirability factor was determined for the composites to compare their structural efficiency. The strength desirability factor for the composites is calculated by the following equation,
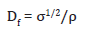
Where, σ and ρ are the strength and density values of the composite material respectively.
The stiffness desirability factor for the composites is calculated by the equation,
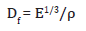
Where, E and ρ are the elastic modulus and density of the composite material respectively [27].
Tribology measurements
The wear and friction analysis were conducted by the standard pin-on-disc method using the Tribometer [Model: Macro PoDTRI- 201 LE, DUCOM instruments (India)]. The study was conducted by preparing the specimens having 10 mm diameter and 15 mm height. The specimens were then rotated against a stainless steel plate (EN 31 hardened up to 62 HRC), with a track diameter of 60mm at an rpm of 300 for 15 minutes. The tribological study was conducted at a load of 3kg at room temperature. The coefficient of friction (COF) was calculated by using the equation,
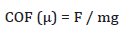
Where F is the force acting on the object, m the mass of the object under motion, and g the acceleration due to gravity (g = 9.81ms-2).
The Specific Wear Coefficient (SWC) was calculated using the relation,
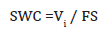
Where, Vi is the wear volume (mm3), F is the normal load acting during testing (N), and S the sliding distance (m) [23].
Water absorption of the composites
The absorption of water by the composites when immersed into two different aqueous environments, which were distilled water and saline water (containing 10% NaCl) were studied. The study was conducted by immersing the specimens in these environments for one-week duration. The specimens, after one week, were withdrawn, wiped dry to remove the surface moisture and were weighed using an electronic balance, accurate to 10-4g for the water absorption measurement. The moisture content, M(t) absorbed by each specimen was calculated from its weight before, m0 and after, mt absorption as follows:
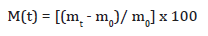
Chemical stability of the composites in acidic and alkaline environments
The experiment was designed to study the effect of aggressive environments surrounding the composite by subjecting them to solutions with acidic (10% HCl solution) and alkaline (10% NaOH solution) pH at room temperature via immersion test. The study was carried out for one-week duration. After the test duration samples were removed, immediately washed in distilled water, wiped to remove surface water drops and then weighed using an electronic balance (accuracy 10-4g) to observe the weight variations before (wo) and after (wt) the immersion. The percentage loss/gain of the treated samples was determined by using the equation,
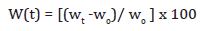
Where, W(t) is weight loss or gain % of the sample, wt is the final weight, and wo is the initial weight of the sample.
Results and Discussion
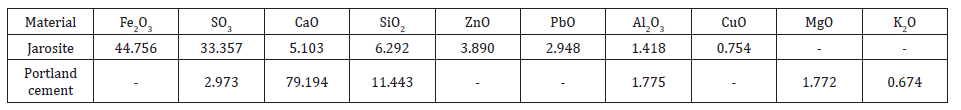
The main constituents of the jarosite, obtained from the industry, and the additive cement are as shown in the Table 2. It can be observed that the major portion in jarosite consists of the oxides of iron, and sulphur (around 77-78wt. % altogether) while the Portland cement consists of the oxides of calcium, and silica (around 90-91wt. % altogether). The presence of heavy metals such as lead, zinc, and copper in jarosite altogether were found to be about 8-9wt. % of the total. The other constituents were the oxides of calcium, silica and aluminum which together constitute around 11-12wt. % of the total. The higher percentage of sulphur can be attributed to the excess use of sulphuric acid, which was used as the catalyst in the jarosite process of metallic zinc extraction. In the case of Portland cement all the other constituents namely oxides of sulphur, aluminum, magnesium, potassium iron together constitute only around 10wt. % of the total. This clearly indicates that it can use as an additive in neutralizing the acidic jarosite waste, as it is found from the earlier studies that lime had been used as a neutralizing agent for the jarosite released as a part of the hydrometallurgical extraction of zinc [30].
Table 2:Major Chemical composition (Wt%) of jarosite and Portland cement.
In Figure 1, X-ray diffraction patterns of Jarosite and Portland cement at room temperature are presented. It can be found that the major mineral phases of jarosite and Portland cement are potassium iron sulphate hydroxide (KFe3(SO4)2(OH)6) and tricalcium silicate (Ca3 SiO5) respectively. However, in jarosite and cement along with the major mineral phases they also constitute the mineral phases of iron sulphate hydrate (2Fe2O3 SO3·5H2 O) and dicalcium silicate (Ca2 SiO4) respectively. Even though the major peaks in jarosite corresponding to potassium iron sulphate hydroxide were identified several of the minor peaks were undecipherable. In cement the presence of a few minor peaks corresponding to calcium magnesium iron silicate were also observed. The mineralogy of jarosite clearly reflects the presence of impurities, which concerning the constitution of mineral waste materials is expected, however the full justification of the occurrence of peaks corresponding to the impurities is beyond the scope of the present work.
Figure 1:XRD spectra of (a) Portland cement, and (b) Jarosite.

From the particle size distribution curve in the Figure 2, for jarosite and cement used in the study, the majority of the particles were found to have a mean particle diameter of 4μm and 14μm respectively. This indicates that both jarosite as well as cement clearly satisfies the criteria of being the particulate phase fillers. The shape and structure of jarosite and Portland cement particles has been presented in Figure 3. It was observed that the shape and structure of jarosite particles were irregular and platy. From Figure 3(a) it can be seen that the variety of particles that exist in jarosite are aggregates, which constitute the collection of strongly bonded particles and do not fully break down under normal processing conditions. On the contrary it can be observed, from Figure 3(b), that in case of cement the particles exist as agglomerates, which in turn constitute the collection of weakly bonded particles.
In comparison to jarosite the cement particle has a non-uniform structure and shape. Further the primary particles appear to form fused irregular aggregates which could be broken down by further processing. The irregularity in the shape of the particles can be attributed to the presence of impurities and the conditions in which they are formed.
Figure 2:Particle size distribution curve of (a) jarosite, and (b) Portland cement.

Figure 3:SEM microstructure of (a) milled jarosite, and (b) Portland cement

Effect of fillers upon the UPR matrix
The interaction of jarosite and cement blended jarosite with the unsaturated polyester resin is examined using the rheology curves from the Figure 4. It was observed that the viscosity of the resin with and without the fillers followed a non-Newtonian flow characteristic showing a shear thinning behavior. The addition of jarosite and cement blended jarosite showed a reduction in the viscosity profile at a filler loading of 15 and 5wt. % in the resin respectively. In case of 15wt % jarosite the viscosity of the resin was found to be well controlled and was lower than that of the neat UPR thus evidencing that jarosite at 15wt % loading in polyester resin formed well dispersed filler - matrix system. Similarly, for cement blended jarosite filler a well dispersed filler - matrix system was found at 5wt % filler loading in the matrix.
Figure 4:Rheology curves showing the effect of (a) jarosite and (b) cement blended jarosite on the viscosity of the unsaturated polyester resin.

The neat UPR has a viscosity of 9.03Pa.s at shear rate up to 96.7 s-1. In comparison to UPR the viscosity of the jarosite, impregnated up to 15wt %, in resin varied between 7Pa.s and 10.5Pa.s and that of cement blended jarosite, impregnated up to 15wt %, in resin varied between 7.3Pa.s and 13.3 Pa.s. This indicates that up to 15wt % of filler concentration the viscosity of the filler - matrix system gets controlled and is nearer to that of the neat polyester resin. The improved rheology of UPR can be ascribed to the morphology of the fillers impregnated in the resin. The dependence of shape factor on the rheological properties of the polymer composite increases as the particle dimension decreases, and the sum of filler - matrix interaction increases [31]. Here the effect of jarosite can be evidenced by implying the lower particle size and platelet like structure which enables the filler-matrix interaction by retaining the stability of the dispersed phase however the presence of cement particles affects the system at higher filler concentration due to its higher dimension and fused irregular structure. Further the dispersion of jarosite above or below the aforementioned level of filler concentration was found to cause the formation of agglomerates leading to the increase in viscosity of the filler - matrix system.
The FTIR analysis of cured polyester reinforced with jarosite and cement blended jarosite are shown in the Figures 5 & 6 respectively. In both the cases the characteristic peaks of unsaturated polyester resin, and jarosite were identified. The prominent absorption peaks at 1728cm-1 and 1736cm-1 in the spectra of polyester reinforced with cement blended jarosite and jarosite respectively corresponds to the stretching of the carbonyl bonds which occur in the neat unsaturated polyester resins. Along with this the absorption peaks at 2960cm-1, 2985cm-1, 1267cm-1, & 1278cm-1 strongly indicates the stretching of C-H bonds and C-O-C bonds which in turn confirms the occurrence of crosslinking between the polyester chains to form the cured resin [31,32]. The strongest peaks, which occur between 1200cm-1 and 1100cm-1, found in the IR spectra of jarosite are due to the stretching vibrations of the SO42-. Moreover, the fundamental vibrations of jarosite due to the combinations SO42- and OH groups were also observed at 2045cm-1 and 1984cm-1 in the jarosite spectra [33,34]. In both the composites the fundamental peaks corresponding to jarosite were identified indicating that the SO42- groups were indisposed to interaction with the polyester resin. The characteristic peaks corresponding to CaO, CaCO3 were identified in the spectra of cement as one flat peak between 1384cm-1 and 1473cm-1. In addition to the characteristic peaks the stretching and bending vibrations H-O-H bonds were also observed at 2521cm-1 and 1788cm-1 in the absorption spectra of cement [35].
Figure 5:FTIR spectra of jarosite, cement, neat polyester (UPR), and UPR with jarosite (PJ).

Figure 6:FTIR spectra of jarosite, cement, neat polyester (UPR), UPR with jarosite-cement (PJC).

The presence of the major peaks in cement was also identified in the band between 1384cm-1 and 1473cm-1 in the polyester reinforced with cement blended jarosite. The polyester reinforced with cement blended jarosite constitutes the peaks corresponding to the vibrations of the SO42- and O-Si-O stretching vibrations at 1168cm-1 in an overlapped manner. The peaks at 3369cm-1 and 3397cm-1 corresponding to the OH stretching vibrations in jarosite and cement respectively were found to be modified in both the polyesters reinforced with jarosite and cement blended jarosite. Thus, from the IR spectra of both the reinforced polyesters it can be observed that the filler phase refrains from any chemical interaction with the matrix.
Effect of initiator upon curing of polyester matrix filled with jarosite as filler
The crucial role played by the initiator on curing behavior of the polyester resin is shown in Figure 7. In a typical reaction as the time proceeds the polymerization reaction accelerates along with the liberation of heat and reaches a maximum temperature which then falls down as the heat is dissipated to the environment. The measure of degree of cure is usually taken as the amount of heat liberated during the reaction while assuming that fractional conversion is proportional to the formation of bonds linking the chains to each other and that formation of each bond releases the same amount of energy [36].
Figure 7:Measured exotherm of UPR-Jarosite composite containing (a) 0.1wt% cobalt Naphthanate and 0.02wt% MEKP (b) 0.1wt% cobalt Naphthanate and 0.05wt% MEKP (c) 0.1wt% cobalt Naphthanate and 0.08wt% MEKP.

It is clearly evident from the exotherm curves in Figure 7, that the raise in peak temperature during the cure increases with the increase in initiator concentration because the rate of polymerization being directly proportional to the increase in the initiator concentration and having a reciprocal relationship with the gel time [37]. However, it is found that with the increase in jarosite concentration the peak temperature begins to fall progressively and reaches a plateau value. This suggests that the curing of matrix depends directly upon the filler content in the matrix and that the fall in peak temperature can be attributed to the absorption of heat generated during the curing process by the filler, which in turn also suggests the delay in reaching gel time with increasing filler concentration.
From Figure 8, it is observed that the change in gelation time with the increasing filler concentration follows a linear path up to a proportionality limit at which the curve deviates from being a straight line. This trend is observed to be the same for the change in gelation time with increasing initiator concentration with respect to increasing filler concentration. The linear path states that gelation time of the matrix is directly proportional to the filler concentration in it, and that the curing behavior of the matrix below the proportionality limit is found to follow high rate of polymerization and low gelation time. It is interesting to note that at the proportionality limit of gelation time, varied with the increasing initiator concentration, has a constant peak temperature of about 47 ℃. This indicates that the onset of cross-linking reactions in the polymer matrix for a particular filler concentration can be optimized with respect to the initiator concentration in such a way that the rate of polymerization remains constant and/or is within the proportionality limit of gelation time. Thus from the Figure 8, it is highly clear that an initiator concentration of about 0.08 wt. % is sufficient enough for the proper curing of matrix with jarosite content almost up to 25wt. %.
Figure 8:Gel time & peak temperature comparison of PJ composites with increasing filler content and initiator concentration (C & E for 0.02wt. % initiator concentration; B & F for 0.05wt. % initiator concentration; A & G for 0.08 wt. % initiator concentration).

Water absorption
The specimens dipped in the two environments were observed for weight change for a one-week duration. The water absorption curves showed in the Figure 9(a) & (b), indicates that both types of composites show good resistance towards water absorption that the neat UPR. The water absorption of the composites in distilled water varied within the range of 4x10-4 to 1x10-4 with PJ30 showing the least water absorption. In case of saline water the water absorption varied between 1.5 x 10-4 and 5 x 10-5 with PJ30 showing the least absorption. This indicates that the water absorption level in the PJ composites decreases with the increase in the filler content the overall absorption in both PJ and PJC composites is very small and the effect seems to impart no damage to the composite.
Figure 9:Variation of water absorption of the (a) PJ & PC composites in distilled water with increasing filler content (b) PJ & PJC composites in saline water with increasing filler content.

From Figure 9(b), it was noticed that both the composites show a huge difference in the absorption behavior in the saline medium. However, the PJ composites show lower absorption tendency in both distilled and saline water, whereas in the case of PJC composites is the complete opposite. This lowering of the absorption tendency of the PJ composites in saline medium can be explained by the slow diffusion process of water into the matrix which occurs due to the presence of large NaCl molecules in water [38]. Meanwhile the PJC composites show increased absorption tendencies due to the presence of cement, which contains tricalcium silicate, dicalcium silicate, tricalcium aluminate, tetracalcium aluminoferrite, and gypsum as the major components, and hence has a greater affinity towards water.
The pH variation of distilled water containing the composites was studied to observe the mobility of ions from jarosite as well as from the composite systems into water which is shown in Figure 10. It was found that in case of jarosite and PJ composites the pH varied from 5.6 to 5.2 during the immersion duration. This is a clear indication that the variation of the pH is caused by the mobility of ions from jarosite. However, the pH of water remained almost equal to 7.5 in case of PJC composites. Moreover, the pH of water is more or less near to neutral for neat polyester during the immersion duration. Thus this indicates the positive effect of the additive cement in reducing the mobility of ions from jarosite in the cement blended jarosite composite over the one without cement.
Figure 10:The pH change of immersion media over time (hours) after composite samples were immersed in that media.

The water absorption pattern of the PJC composites in two environments was found to follow low absorption tendency up to 15 wt. % of the filler content after which steady rise was observed. However, the PJ composites were found to have a very low water absorption tendency than the neat UPR and PJC composite in both environments. This clearly indicating the better adhesion between the matrix and jarosite filler along with lower affinity towards hydroxyl groups.
Chemical stability in acidic and alkaline environments
The data relating to the chemical stability of the composites were presented in Figure 11 for the various PJ and PJC composites. The analysis of the result shows the weight loss of composites in comparison with the neat UPR when immersed in the selected environments. From Figure 11(a) & (b) it is clearly evident that the composites are relatively less stable in the alkaline environment than in the acidic environment. In addition, Figure 12 shows the formation of cracks, at a higher filler loading, as well as the discoloration of the composites in the alkaline medium which in turn suggests the lower stability of composites in alkaline medium. The formation of cracks in the composites can possibly be due to the stress corrosion cracking that occurs in polymers as a result of degradation by the aggressive environments and the discoloration indicates the chemical attack of the medium upon composite.
Figure 11:Variation of weight percent of (a) PJ & PJC composites with increasing filler concentration immersed in alkaline environment (b) PJ & PJC composites with increasing filler concentration immersed in acidic environment

Figure 12:Color change of PJ & PJC composites after immersion under alkaline environment in comparison with acidic & aqueous environment.

It is also observed from the graphs that in case of PJC composites, the stability in the acidic medium is much higher than the PJ composites. This suggests that the additive cement in the composites has a positive effect upon their acid stability. This is however not observed in case of an alkaline medium. This chemical study thus indicates that the PJC composites, in comparison to PJ composites, remain resistant to the aggressive environments to which it was subjected. It is also noted that up to 15wt. % of jarosite in both the ccomposite in both mediums remain equally stable. It is of course necessary that long-term studies are needed to confirm this observation over a longer duration of time.
Figure 13(a) shows the compressive strength of the two composites with respect to the loading of filler content. From Figure 14 it was observed that neat polyester resin has an average compression strength value of 58.87MPa, which upon reinforcement was found to increase up to 89.47MPa for the PJC composites and up to 81.21MPa for the PJ composites. The enhancement of the compressive strength was found to be steadily increasing up to 15wt. % of filler content for both the composites. This trend was then observed to fall steadily, as shown in Figure 13(a). The increase in the improvement of compressive strength for neat polyester resin with the increasing filler content up to 15 wt. % can be accounted as 7%, 19% & 38% for 5, 10 & 15wt.% of filler content in PJ composites, and 17%, 31% & 52% for 5, 10 & 15wt.% of the filler content in PJC composites respectively. This shows that the addition of 20 parts of cement with 80 parts of jarosite improves the compressive strength of the matrix by a maximum of 10% compared to the use of jarosite alone as the filler. The increase in the compression strength of the composites can be attributed to the fact that homogeneous distribution and interfacial bonding by the reinforcement can lead to improvement in the mechanical strength of composites.
Figure 13:(a) Compressive strength of PJ &PJC composites and (b) comparitive analysis of stress-strain curve for the PJ15 & PJC15 composites with respect to neat UPR.

Figure 14:Fractured images of Neat Polyester, Polyester-Alumina, PJ15 composite, & PJC15 composite.

From Tables 3 & 4 it was observed that the average values of compression strength, toughness, hardness as well as the desirability was overall enhanced by the additive cement for the PJC composites. Moreover, the composites were found to show maximum toughness at 10wt.% of filler loading, however the structural efficiency of the PJC15 composite was much higher than the other wt.% of PJC and PJ composites. The fractured images of the PJ15 and PJC15 were shown in Figure 14. This shows that the composite has undergone ductile fracture and the buckling of the PJ15 & PJC15 composites under ductile fracture can be observed in both the composites. The brittle fracture in a typical polyesteralumina reinforced composite as well as in neat polyester resin were also shown for better comparison. The stress-strain graph shown in Figure 13(b) also confirmed that the addition of fillers transformed the brittle nature of polyester matrix into a ductile one. However, this is found to be true only up to 15wt. % of filler content in the matrix as above this wt. % the proper packing of the filler inside the matrix seems to be improper. This can be attributed to the decrease in adhesion between the matrix and the filler particles as a result of weak cross-linking at filler-matrix interface which in turn leads to the low transfer of compressive stress [35].
Table 3:Comparison of the mechanical & tribological properties of the PJ composite with increasing filler content.
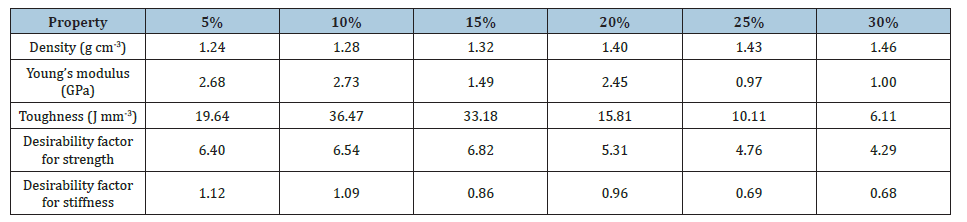
Table 4:Comparison of the mechanical & tribological properties of the PJC composite with increasing filler content.
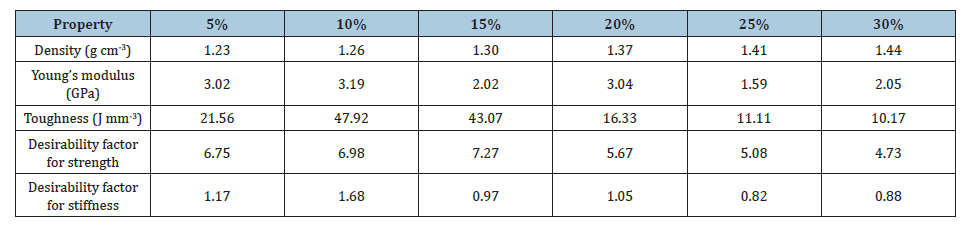
Tribology
The data obtained relating to the Specific Wear Rate (SWR) and Coefficient of Friction (COF) of PJ & PJC composites with respect to increasing filler content in the polyester matrix were presented in Figure 15. It is observed that the wear rate & coefficient of friction of PJ composites increases with the increase in filler concentration in the matrix. The increase in the wear rate and friction coefficient of the PJ composites can be attributed to the effects of loose damaged composite particles containing jarosite at the contact interface. This loose debris gives rise to more loose particles which in turn increases the abrasive force leading to increased frictional force and wear rate of the composites, also known as the three body abrasion mechanism [39].
Figure 15:Comparison of coefficient of friction & specific wear rate of PJ vs. PJC with respect to filler loading.

In case of the PJC composites, Figure 15 shows that the frictional coefficient steadily increases. This increase can also be attributed to the three body abrasion wear process. However, the coefficient of friction for PJC composites was found to have lower values in comparison with PJ composites. In case of neat polyester, the adhesive wear turns out to be the primary cause for material removal. The frictional coefficients for both PJ & PJC composites varied between 0.48-0.67, where PJC & PJ showed the least frictional coefficient of 0.498 & 0.512 respectively for 5wt. % of filler, but the wear rates of PJC composites were found to follow a decreasing trend. However, from Figure 15 it can be observed that in comparison to PJ composites the wear rate of PJC composites tends to remain almost stable. This indicates the positive effect of cement up on the PJC composites which along with improving the strength of the composites, also leads to a decrease in wear rate.
The microstructure analysis of the wear surfaces for the PJ and PJC composites were carried out under SEM and are shown in Figure 16. The changes in wear rate with respect to filler concentration for the PJ and PJC composites can be explained by the changes in the roughness of the wear surface caused as a result of the accumulated wear debris in the counter face [40]. In case of PJ composites, it can be seen that with increasing filler content the composite undergoes more and more plastic deformation under the combined stresses of compression and shear resulting in large wear rates. There are broad wear marks along with deep pull outs in PJ composites in comparison to that of PJC composites. The overall mechanical and tribological properties of both the Jarosite and jarosite/cement particulate fillers reinforced polyester matrix composite is summarized in the Tables 3 & 4.
Figure 16:SEM images (x 400) of the worn surfaces of neat polyester, PJ composites, and PJC composites after tribological analysis at a load of 29.8 N for 15 minutes.

From the Table 3, it can be found that the addition of jarosite as well as jarosite-cement fillers have considerably reduced the wear rate as well as the frictional coefficient of the UPR matrix, up to 10wt. %, indicating that the soft reinforcing phase in the brittle UPR matrix enables in reducing the wear rates along with imparting the matrix with a certain amount of ductility in the contact region. The presence of loose debris can be clearly seen at higher filler loading of jarosite in PJC composite while higher erosion due to wear is progressively evident in the PJ composites as well as in the neat UPR resin.
Conclusion
The incorporation of jarosite as filler phase, generated as the byproduct from the zinc industries, in the polyester resin has turned out to be a better way for its disposal as well as its utilization in a significant manner. The results from the present study revealed that the compression strength of the polyester resin has been enhanced up to 52% with the addition of cement-blended jarosite. The polyester matrix can be cured at a constant peak temperature of around 47-50 ℃; even with the loading of filler content up to 30% a well-dispersed filler-matrix system is achievable only up to the addition of 5-15% of filler content. Ultimately the cement blended jarosite composite exhibited a significant improvement in the specific wear rate as well as the coefficient of friction than jarosite composites at a constant load and the existence of stronger filler-matrix interaction in the cement blended jarosite composites was proved by tribological analysis. Further, the composites showed good resistance to degradation from humidity and the cement-blended jarosite composites maintained the water pH of 7.5, implying that cement enables in controlling the mobility of ions from the jarosite embedded in the matrix. The composites had moderate resistance to acid or alkali attack; however, cementblended jarosite composites exhibited better resistance than the jarosite-based composites in acid medium. Overall, both the filler phases influence the mechanical properties, and the composites have good structural efficiency. Moreover, the addition of cement facilitates additional strength as well as enables immobilization of the jarosite within the matrix along with enhancing the overall properties of the composite.
References
- Fu SY, Feng XQ, Lauke B, Mai YW (2008) Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate-polymer composites. Composites Part B: Engineering 39(6): 933-961.
- Pendhari SS, Kant T, Desai YM (2008) Application of polymer composites in civil construction: A general review. Composite structures 84(2): 114-124.
- Afzal A, Kausar A, Siddiq M (2020) Role of polymeric composite in civil engineering applications: a review. Polymer-Plastics Technology and Materials 59(10): 1023-1040.
- Rodriguez EL (1987) Particulate filled polyester composites. Journal of materials science letters 6(11): 1280-1282.
- Spanoudakis J, Young RJ (1984) Crack propagation in a glass particle-filled epoxy resin. Journal of Materials Science 19(2): 473-486.
- Dekkers MEJ, Heikens D (1983) The effect of interfacial adhesion on the mechanism for craze formation in polystyrene-glass bead composites. Journal of Materials Science 18(11): 3281-3287.
- Sharma RP, Kumar M (2020) Mechanical and tribological performance of polymer composite materials: a review. In Journal of Physics: Conference Series 1455(1): 012033.
- El-Sayed AA, El-Sherbiny MG, Abo-El-Ezz AS, Aggag GA (1995) Friction and wear properties of polymeric composite materials for bearing applications. Wear 184(1): 45-53.
- Friedrich K, Zhang Z, Schlarb AK (2005) Effects of various fillers on the sliding wear of polymer composites. Composites science and technology 65(15-16): 2329-2343.
- Alaei MH, Mahajan P, Brieu M, Kondo D, Rizvi SJA, et al. (2013) Effect of particle size on thermomechanical properties of particulate polymer composite. Iranian Polymer Journal 22(11): 853-863.
- Benny Cherian A, Thachil ET (2001) Toughening studies of an unsaturated polyester resin using maleated elastomers. Progress in Rubber and Plastics Technology 17(4): 205-224.
- Dekkers MEJ, Heikens D (1983) The effect of interfacial adhesion on the tensile behavior of polystyrene–glass‐bead composites. Journal of Applied Polymer Science 28(12): 3809-3815.
- Bahadur S, Zheng Y (1990) Mechanical and tribological behavior of polyester reinforced with short glass fibers. Wear 137(2): 251-266.
- Sen S, Nugay N (2001) Tuning of final performances of unsaturated polyester composites with inorganic microsphere/platelet hybrid reinforcers. European Polymer Journal 37(10): 2047-2053.
- Madugu IA, Abdulwahab M, Aigbodion VS (2009) Effect of iron fillings on the properties and microstructure of cast fiber–polyester/iron filings particulate composite. Journal of Alloys and Compounds 476(1-2): 807-811.
- Patnaik A, Satapathy A, Mahapatra SS, Dash RR (2009) A comparative study on different ceramic fillers affecting mechanical properties of glass-polyester composites. Journal of Reinforced Plastics and Composites 28(11): 1305-1318.
- Datta J, Włoch M (2014) Influence of selected submicron inorganic particles on mechanical and thermo-mechanical properties of unsaturated polyester/glass composites. Journal of Reinforced Plastics and Composites 33(10): 935-941.
- Kumar GN, Reddy YM, Reddy KH (2015) Synthesis and characterization of iron oxide nanoparticles reinforced polyester/nanocomposites. International Journal of Scientific and Research Publications 5(8): 1-13.
- Gull N, Khan SM, Munawar MA, Shafiq M, Anjum F, et al. (2015) Synthesis and characterization of zinc oxide (ZnO) filled glass fiber reinforced polyester composites. Materials & Design 67: 313-317.
- Ribeiro MCS, Sousa SPB, Nóvoa PRO (2015) An investigation on fire and flexural mechanical behaviors of nano and micro polyester composites filled with SiO2 and Al2O3 Materials Today: Proceedings 2(1): 8-19.
- Patel VK, Dhanola A (2016) Influence of CaCO3, Al2O3, and TiO2 microfillers on physico-mechanical properties of Luffa cylindrica/polyester composites. Engineering Science and Technology, an International Journal 19(2): 676-683.
- Munawar MA, Khan SM, Gull N, Shafiq M, Islam A, et al. (2016) Fabrication and characterization of novel zirconia filled glass fiber reinforced polyester hybrid composites. Journal of Applied Polymer Science 133(27).
- Selvam R, Ravi S, Raja R (2017) Fabrication of SiC particulate reinforced polyester matrix composite and investigation. In IOP Conference Series: Materials Science and Engineering 197(1): 012052.
- Islam M, Ar-Rashid H, Islam F, Karmaker N, Koly FA, et al. (2019) Fabrication and characterization of e-glass fiber reinforced unsaturated polyester resin based composite materials. Nano Hybrids and Composites24: 1-7.
- Mishra D, Mohapatra S, Satapathy A (2019) A comparative study of characterization and water absorption behaviour of polyester composites with inorganic and organic fibers. Materials Today: Proceedings 19: 289-295.
- Hsing HJ, Wang FK, Chiang PC, Yang WF (2004) Hazardous wastes transboundary movement management: a case study in Taiwan. Resources, Conservation and Recycling 40(4): 329-342.
- Mymrin VA, Ponte HA, Impinnisi PR (2005) Potential application of acid jarosite wastes as the main component of construction materials. Construction and Building Materials 19(2): 141-146.
- Asokan P, Saxena M, Asolekar SR (2006) Hazardous jarosite use in developing non-hazardous product for engineering application. Journal of Hazardous Materials 137(3): 1589-1599.
- Le Saoût G, Kocaba V, Scrivener K (2011) Application of the Rietveld method to the analysis of anhydrous cement. Cement and Concrete Research 41(2): 33-148.
- Gupta C, Prasad A (2018) Variables controlling strength of lime stabilized jarosite waste. International Journal of Geo-Engineering 9(1): 1-15.
- Kuzdzal E, Cichy B, Dulik S (2016) Morphological properties of fillers for polymeric materials; the influence on rheological properties of compositions with unsaturated polyester resin. Chemik 70(4): 189-192.
- Paauw M, Pizzi A (1993) Completion of unsaturated polyesters analysis by FTIR. Journal of Applied Polymer Science 48(5): 931-934.
- Bishop JL, Murad E (2015) The visible and infrared spectral properties of jarosite and alunite. American Mineralogist 90(7): 1100-1107.
- Hunt GR (1971) Visible and near-infrared spectra of minerals and rocks: IV. Sulphides and sulphates. Modern Geology 3: 1-14.
- Nasrazadani S, Springfield T (2014) Application of Fourier transform infrared spectroscopy in cement Alkali quantification. Materials and Structures 47(10): 1607-1615.
- Cook WD, Lau M, Mehrabi M, Dean K, Zipper M (2001) Control of gel time and exotherm behaviour during cure of unsaturated polyester resins. Polymer International 50(1): 129-134.
- Yang YS, Lee LJ (1987) Rheokinetic studies of unsaturated polyester resins. Polymer Process Engineering 5(3-4): 327-356.
- Shah V (2020) Handbook of plastics testing and failure analysis. John Wiley & Sons.
- Kato K (2000) Wear in relation to friction - a review. Wear241(2): 151-157.
- El-Tayeb NS, Gadelrab RM (1996) Friction and wear properties of E-glass fiber reinforced epoxy composites under different sliding contact conditions. Wear 192(1-2): 112-117.
© 2025 © Ananthakumar S. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






