- Submissions

Full Text
Research & Development in Material Science
Synthesis and Applications of Metal Organic Frameworks
Anindita Chakraborty1,2 and Himadri Acharya2*
1Institue of Chemical Technology, Marathwada Campus, India
2Department of Chemistry, Assam University, India
*Corresponding author: Himadri Acharya, Centre for Soft Matters, Department of Chemistry, Assam University, Silchar-788011, Assam, India
Submission: June 12, 2023;Published: June 26, 2023

ISSN: 2576-8840 Volume 19 Issue 1
Abstract
This mini review describes the Metal Organic Framework (MOF) as an emerging class of organic-inorganic materials significant for potential in catalysis, adsorption, gas storage, sensing and biomedical applications. The concept of linkers, secondary building units of metal organic frameworks, their various synthetic strategies and selective prospective applications are reviewed.
Keywords:Metal organic framework; Linkers; Synthesis; Applications
Introduction
Metal Organic Frameworks (MOFs) are the important class of porous materials that comprise of organic-inorganic building blocks, in which organic components are called linkers and inorganic parts are called nodes or Secondary Building Units (SBUs). Linkers are usually bi- or multi-dentate organic ligands and SBUs are inorganic metal ions or metal-oxo clusters [1]. MOFs are also known as three-dimensional porous coordination polymers (PCPs). MOFs have attained considerable research interest due to their tunable pore size, enhanced surface area, low framework density, high thermal and chemical stability and diverse functionality, which enable them to stand with higher impacts over other porous materials [2].
From last few years, MOFs have been considered as the structurally sound organic-inorganic hybrid materials due to their vast structural diversity and ease of synthesis. Therefore, researchers are frequently developing different types of MOFs and till date more than 20,000 MOFs have been rationally designed with advanced topology, which are sincerely taking part in various fields of applications such as catalysis, sensing, adsorption and separation, gas storage etc [2].
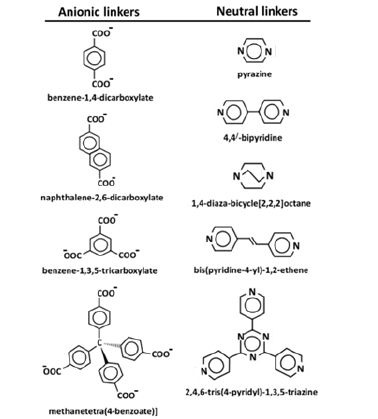
MOFs consist of organic linkers and inorganic metal/metal-oxo clusters (SBUs). Self-assembly of linkers and SBUs gives rise to three dimensional arrays, in which linkers and SBUs are joined together by covalent bonds. The pore dimension and surface area of MOFs can be frequently tailored by altering the length of organic linkers or coordination geometry of SBUs. Thus, designing of great variety of MOFs can be possible with different void structures and diverse structural geometries. The common rigid organic linkers include di-, tri-, tetra-topic carboxylate groups, neutral nitrogen heterocycles etc. Terephthalate (benzene-1,4-dicarboxylate), one of the most common di-topic organic linkers is linear in shape. It has fascination to interact with paddle-wheel, octahedral or trigonal-prismatic metal/meta-oxo clusters. Similarly, trigonal planer tri-topic organic linker, for example trimesate (benzene-1,3,5-tricarboxylate) or tetrahederal/tetra-topic organic linker, for example methanetetra (4-benzoate) are also able to form multi-dimensional MOFs structures with paddle-wheel, octahedral, trigonal-prismatic, cubical, square planar, hexagonal bipyramidal SBUs [3]; (Figure 1).
Figure 1:Represents the structures of a few commonly used rigid organic linkers in MOFs synthesis
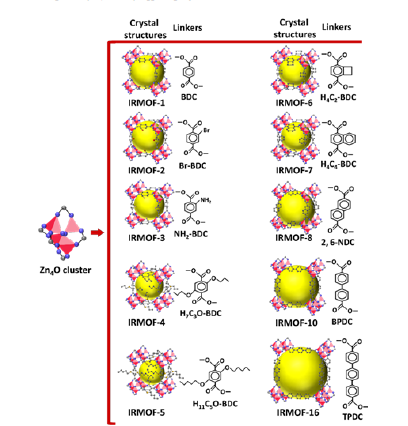
The variation of functional groups in linkers or change in linkers’ length lead to the formation of MOFs with tunable structure, geometry, pore dimension and surface area. For example, terephthalate, the well-known di-topic organic linker, when connected to Zn4O clusters, leads to the formation of IRMOF-1 (MOF-5). But, the replacement of one or two hydrogen atoms with different functional groups such as -Br, -NH2, -OC3H7, -OC5H11, -C2H4 (cyclobutyl) and -C4H4 (fused benzene) in phenyl ring of terephthalate results in the formation of IRMOF-2, IRMOF-3, IRMOF-4, IRMOF-5, IRMOF-6, IRMOF-7 respectively. Notably, the variation of functional groups in linkers leads to the disparity of surface area and pore size of IRMOFs. Additionally, increase in linker’s length leads to increase in pore dimension [4], as represented in Figure 2.
Figure 2:Crystal structures of various MOFs synthesized using different carboxylate ligands (the yellow spheres represent the voids).
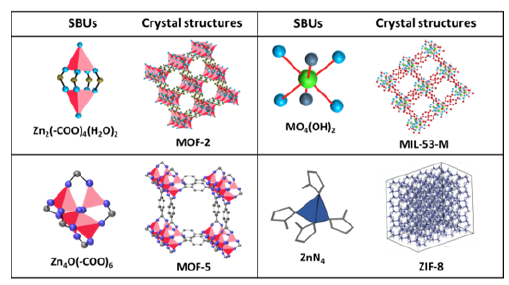
It is worth mentioning that fabrication of MOFs not only depends on linkers but the structure of secondary building units strictly regulate the specific design of MOFs. In the context of permanent porosity, O. M. Yaghi and his co-workers first developed three dimensional, permanently porous, rigid MOF structure namely MOF-5, which contains octahedral Zn-based metal clusters Zn4O and BDC ligands [5]. Thereafter, a number of porous, rigid MOFs structures were developed with various SBUs. For example, MOF-199 (HKUST-1) can be developed by using tetrahedral Cu(II) metal sites [Cu2(-COO)4] and trimesic acid. Cu(BDC) MOFs are fabricated by connecting the Cu(II) dimers with BDC linkers in bidentate bridging fashion [6]. MIL-n series (where, MIL=Matériaux de I′Institut Lavoisier) were first discovered by Ferey and coworkers in 2002. MIL-53-M (M = trivalent metal ions such as Al3+ / Fe3+ / Cr3+ etc.) were fabricated by corner sharing the octahedral MO4(OH)2 clusters with terephthalate linkers [7]. MIL-88B (B implies BDC ligands) and MIL-101 can be synthesized using trigonal prism [M3O(OOCR)6L3]n+ (L implies terminal ligand viz. H2O or Cl−) SBUs, coordinated with six terephthalate ligands [8]. Zeolite type MOFs such as ZIFs can be built up by connecting the tetrahedrally coordinated MN4 (where, M=Zn/Co) clusters with ditopic imidazolate linkers [9]. Geometries of a few secondary building units and the corresponding crystal structures of MOFs are shown in Figure 3.
Figure 3:A few representative SBUs and corresponding crystal structures of different MOFs.
Synthesis of metal organic frameworks
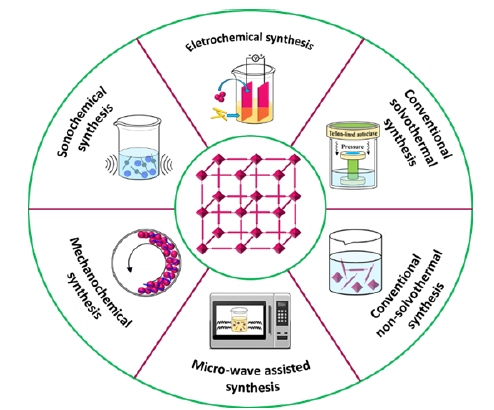
Metal organic frameworks are synthesized by various methods as shown in Figure 4. The most common methods are conventional method, microwave assisted synthesis, mechanochemical synthesis, sonochemical synthesis and electrochemical synthesis.
Figure 4:Various methods of MOFs synthesis.
Conventional method of MOFs synthesis: MOF synthesis in conventional method usually refers to the method that involves electric heating during synthesis. Conventional method includes solvothermal and non-solvothermal processes. In solvothermal process, the reaction is carried out in sealed container and the temperature of the reaction mixture is raised above the boiling point of the solvent. In this process, high pressure is generated in the closed container that predominantly regulates the reaction. In the closed reaction chamber, the linkers interact with metal ions, subsequently nucleation occurs followed by growth that lead to the formation of highly crystalline MOFs structures. There are numerous metal organic frameworks synthesized using this approach. Highly crystalline Zn-based MOFs were synthesized via solvothermal method [10]. Two dimensional fluorinated metal organic frameworks such as F-MOF-4, Cu-F-MOF-4B, Zn-F-MOF- 4B were synthesized solvothermally [11]. Highly crystalline MIL- 101(Cr) was synthesized via hydrothermal approach [12].
Whereas, in non-solvothermal process, the reaction is carried out at room temperature or the temperature of the reaction can be fixed at boiling point of the solvent. Room temperature nonsolvothermal approach was adopted for the synthesis of various MOFs such as Cu-BTC MOF, UiO-66, Zn-based metal organic frameworks etc [13,14]. In non-solvothermal approach, the rate of nucleation and growth of metal organic frameworks can be tuned by changing the reaction temperature or evaporation of the solvent at slightly higher temperature.
Microwave assisted MOFs synthesis: In microwave (MW) assisted MOF synthesis, electromagnetic waves interact with solid or liquid materials, which results in high molecular orientation in solvent or reactant materials. These lead to the elevation of temperature in the reaction medium. In MW assisted synthesis, the high and homogeneous temperature can be maintained throughout the reaction medium. The solvents used in MW assisted synthesis can be selective, so that the electromagnetic waves can interact strongly with the reaction medium. Cr-based MIL-100 metal organic framework was first synthesized by this approach [15]. Later on, IRMOF-1, 2, 3 were synthesized using this process [16].
Mechanochemical synthesis of MOFs: In mechanochemical synthesis, the mechanical force breaks the inter-molecular bonds and simultaneously chemical transformation occurs in the reactant molecules to yield the product. The entire process occurs in solventfree condition, therefore, the process is very much eco-friendly. The first MOF synthesized in this process was three dimensional Cubased MOF [Cu(INA)2], where isonicotinic acid was used as linker. After the first report, HKUST-1, MOF-14, [Zn(EIm)2] MOFs were synthesized by mechanochemical process [17-19].
Sonochemical synthesis of MOFs: In sonochemical process, high energy ultrasonic vibrations are applied to the reaction mixture. This energy-efficient process is used to synthesize a number of metal organic frameworks. The first MOF, synthesized using this approach was [Zn3(BTC)2. 12H2O] [20]. Sonochemical approach was also employed to synthesize MOF-5, HKUST-1, ZIF-8 etc [21-23].
Electrochemical synthesis of MOFs: In electrochemical synthesis, metal ions are allowed to pass through the anodic dissolution to the reaction medium, where they interact with dissolved linkers to form metal organic frameworks. The first electrochemically synthesized MOF was Cu-BTC MOF [24]. Thereafter, Ni-BTC, HKUST-1, ZIF-8, MIL-100(Al), MIL-53(Al), and NH2-MIL-53(Al), MOF-5 were also framed with electrochemical approach [25-27]..
Applications of metal organic frameworks
Metal organic frameworks have attained remarkable research interest due to their wide-spread applications in the field of catalysis, sensing, adsorption and separation, biological applications etc., as shown in Figure 5.
Figure 5:Various applications of metal organic frameworks.
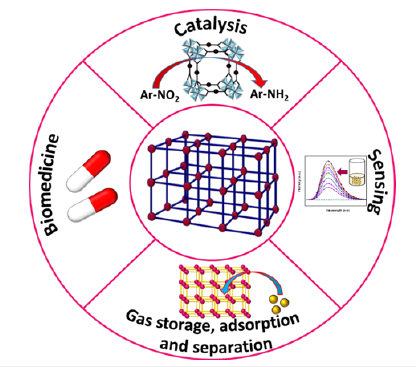
Gas storage, adsorption and separation: MOFs are crystalline solids having permanent porosity and large surface area, for which they are capable of storing various gas molecules, solids and liquids within their structures. MOF with divalent metal ions such as HKUST-1 exhibits excellent porosity, therefore, they can uptake methane (CH4) gas molecules [28]. PCN-250(Fe2M) MOFs, where, M=Fe, Co, Ni, Mn and Zn are used in CH4 storage applications [29]. The flexible MOFs are advantageous over rigid MOFs in gas storage performance. For example, MIL-53(Al) can uptake huge amount of CH4 gas at room temperature [30]. MOF-177 and was reported for CO2 storage application [31]. MOFs are also proficient to adsorb toxic chemicals from water. For example, UiO-66, PCN-222 were used as toxic dye adsorbents [32,33]
Catalysis: MOFs act as very good catalyst in many heterogeneous chemical reactions. The catalytic activity of MOFs is directly correlated to metal centers, as the coordinatively unsaturated secondary building units act as Lewis acids for many chemical reactions. For example, MIL-100(Fe) was used as Lewis acid catalyst in Friedel-Crafts reactions [34], regioselective ring-opening reactions of epoxides [35], Claisen-Schmidt condensation reactions [36], Knoevenagel condensation reaction [37], cyanosilylation reaction [38], etc. UiO-66 can be used in aldol condensation reaction [39]. Besides these, MOFs can act as promising light harvesting materials in photocatalysis. For example, PCN-22 was used as photo catalyst in light driven alcohol oxidation reaction [40]. MIL- 101(Fe) also acts as excellent photocatalyst in photocatalytic water oxidation reaction [41].
Sensing: MOFs are used as excellent chemical sensors. For instance, water soluble Cd-based MOF [Cd2(TIB)2(BDA)2] was used as chemical sensor for the detection of ketones in aqueous medium [42]. Fluorescent metal organic framework, MIL-53(Al) was used for the detection of Fe3+ ions in aqueous solution [43]. Antibiotics and explosives in water could be detected by Zr-based MOFs such as Zr6O4(OH)8(H2O)4(CTTA)8/3 and Zr6O4(OH)8(H2O)4(CTTA)8/3 [44]. Toxic heavy metals in water can be detected by UiO-66 [45]. Fumarate based RE-fcu-MOF thin film has the ability for selective detection of H2S gas [46].
Biomedical applications: In the field of biomedicine, MOFs play a vital role owing to their well-defined structures, tunable pore size and large specific surface area. MOFs with non-toxic metal-sites were used as host matrices to incorporate biologically active compounds such as drugs, enzymes etc [28]. The pioneering work on MOF based drug delivery was carried out by Férey et al. [47] in 2005. Based on Férey’s work, lots of research works are being continued to further explore the MOF’s proficiency in the field of targeted drug delivery systems. For example, flexible metal organic frameworks MIL-53(Cr, Fe) were used for in vitro release of ibuprofen [48]. ZIF-8 was also used as host matrix for the release of anticancer drug doxorubicin [49]. Non-conventional anti-cancer drug such as [Ru(p-cymene)Cl2(pta)] (RAPTA-C) was successfully released into SBF solution by Ni-based MOF (CPO-27-Ni) [50].
Conclusion
This review endeavored to the concepts, various synthetic strategies and applications of MOFs. The MOFs can be prepared by Conventional methods, microwave assisted, sonochemical, mechanochemical and electrochemical approaches. The structural analysis established the MOF crystal structure. These MOFs have been successfully used for various applications such as catalysis, adsorption, gas storage, sensing, and biomedical applications.
Conflict of Interest
There are no conflicts to declare.
References
- Rosi NL, Eddaoudi M, Kim J, O'Keeffe M, Yaghi OM (2002) Advances in the chemistry of metal-organic frameworks. Cryst Eng Comm 4: 401-404.
- Furukawa H, Cordova KE, O’Keeffe M, Yaghi OM (2013) The chemistry and applications of metal-organic frameworks. Science 341: 1230444.
- Lu W, Wei Z, Gu ZY, Liu TF, Park J, et al. (2014) Tuning the structure and function of metal–organic frameworks via linker design. Chem Soc Rev 43: 5561-5593.
- Eddaoudi M, Kim J, Rosi N, Vodak D, Wachter J, et al. (2002) Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 295: 469-472.
- Kalmutzki MJ, Hanikel N, Yaghi OM (2018) Secondary building units as the turning point in the development of the reticular chemistry of MOFs. Sci Adv 4: 9180.
- Carson CG, Hardcastle K, Schwartz J, Liu X, Hoffmann C, et al. (2009) Synthesis and structure characterization of copper terephthalate metal-organic frameworks. Eur J Inorg Chem 2009(16): 2338-2343.
- Millange F, Serre C, Ferey G (2002) Synthesis, structure determination and properties of MIL-53as and MIL-53ht: the first Criiihybrid inorganic–organic microporous solids: Criii(OH)·{O2C–C6H4–CO2}·{HO2C–C6H4–CO2H}x. Chem Commun 8: 822-823.
- Carson F, Su J, Prats AEP, Wan W, Yun Y, et al. (2013) Framework isomerism in vanadium metal-organic frameworks: MIL-88B (V) and MIL-101 (V). Cryst Growth Des 13: 5036-5044.
- Venna SR, Jasinski JB, Carreon MA (2020) Structural evolution of zeolitic imidazolate framework-8. J Am Chem Soc 132: 18030-18033.
- Nazari Z, Taher MA, Fazelirad H (2017) A Zn based metal organic framework nanocomposite: synthesis, characterization and application for preconcentration of cadmium prior to its determination by FAAS. RSC Adv 7: 44890-44895.
- Pachfule P, Das R, Poddar P, Banerjee R (2011) Solvothermal synthesis, structure, and properties of metal organic framework isomers derived from a partially fluorinated link. Cryst Growth Des 11: 1215-1222.
- Yang LT, Qiu LG, Hu SM, Jiang X, Xie AJ, et al. (2013) Rapid hydrothermal synthesis of MIL-101 (Cr) metal-organic framework nanocrystals using expanded graphite as a structure-directing template. Inorg Chem Commun 35: 265-267.
- Getachew N, Chebude Y, Diaz I, Sanchez MS (2014) Room temperature synthesis of metal organic framework MOF-2. J Porous Mater 21: 769-773.
- Zhang H, Duan C, Li F, Xi H (201) Rapid room-temperature synthesis of hierarchical porous metal organic frameworks. AIP Conf Proc 1971: 020023.
- Jhung SH, Lee JH, Chang JS, San J (2005) Microwave synthesis of a nanoporous hybrid material, chromium trimesate. Bull Korean Chem Soc 26: 880-881.
- Ni Z, Masel RI (2006) Rapid production of metal - organic frameworks via microwave-assisted solvothermal synthesis. J Am Chem Soc 128: 12394-12395.
- Pichon A, James SL (2008) An array-based study of reactivity under solvent-free mechanochemical conditions-insights and trends. Cryst Eng Comm 10: 1839-1847.
- Klimakow M, Klobes P, Thünemann AF, Rademann K, Emmerling F (2010) Mechanochemical synthesis of metal - organic frameworks: a fast and facile approach toward quantitative yields and high specific surface areas. Chem Mater 22: 5216-5221.
- Beldon PJ, Fábián L, Stein RS, Thirumurugan A, Cheetham AK, et al. (2010) Rapid room‐temperature synthesis of zeolitic imidazolate frameworks by using mechanochemistry. Angew Chem Int Ed 49: 9640-9643.
- Qiu LG, Li ZQ, Wu Y, Wang W, Xu T, et al. (2008) Facile synthesis of nanocrystals of a microporous metal-organic framework by an ultrasonic method and selective sensing of organoamines. Chem Commun 31: 3642-3644.
- Son WJ, Kim J, Kim J, Ahn WS (2008) Sonochemical synthesis of MOF-5. Chem Commun 47: 6336-6338.
- Schlesinger M, Schulze S, Hietschold M, Mehring M (2010) Evaluation of synthetic methods for microporous metal-organic frameworks exemplified by the competitive formation of [Cu2 (btc)3 (H2O)3] and [Cu2(btc)(OH)(H2O)]. Microporous Mesoporous Mater 132: 121-127.
- Li ZQ, Qiu LG, Su T, Wu Y, Wang W, et al. (2009) Ultrasonic synthesis of the microporous metal-organic framework Cu3 (BTC)2 at ambient temperature and pressure: an efficient and environmentally friendly method. Mater Lett 63: 78-80.
- Mueller U, Schubert M, Teich F, Puetter H, Schierle-Arndt K, et al. (2006) Metal-organic frameworks-prospective industrial applications. J Mater Chem 16: 626-636.
- Jabarian S, Ghaffarinejad A (2019) Electrochemical synthesis of NiBTC metal organic framework thin layer on nickel foam: An efficient electrocatalyst for the hydrogen evolution reaction. J Inorg Organomet Polym Mater 29: 1565-1574.
- Joaristi AM, Alcañiz JJ, Crespo PS, Kapteijn F, Gascon J (2012) Electrochemical synthesis of some archetypical Zn2+, Cu2+, and Al3+ Metal Organic Frameworks. Cryst Growth Des 12: 3489-3498.
- Li WJ, Tu M, Cao R, Fischer RA (2016) Metal-organic framework thin films: electrochemical fabrication techniques and corresponding applications & perspectives. J Mater Chem A 4: 12356-12369.
- Yuan S, Feng L, Wang K, Pang J, Bosch M, et al. (2018) Stable metal-organic frameworks: design, synthesis, and applications. Adv Mater 30: 1704303.
- Feng D, Wang K, Wei Z, Chen YP, Simon CM, et al. (2014) Kinetically tuned dimensional augmentation as a versatile synthetic route towards robust metal-organic frameworks. Nat Commun 5: 5723.
- Bourrelly S, Llewellyn PL, Serre C, Millange F, Loiseau T, et al. (2005) Different adsorption behaviors of methane and carbon dioxide in the isotypic nanoporous metal terephthalates MIL-53 and MIL-47. J Am Chem Soc 127: 13519-13521.
- Millward AR, Yaghi OM (2005) Metal - organic frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature. J Am Chem Soc 127: 17998-17999.
- Mohammadi AA, Alinejad A, Kamarehie B, Javan S, Ghaderpoury A, et al. (2017) Metal-organic framework Uio-66 for adsorption of methylene blue dye from aqueous solutions. Int J Environ Sci Technol 14: 1959-1968.
- Li H, Cao X, Zhang C, Yu Q, Zhao Z, et al. (2017) Enhanced adsorptive removal of anionic and cationic dyes from single or mixed dye solutions using MOF PCN-222. RSC Adv 27: 16273-16281.
- Horcajada P, Surble S, Serre C, Hong DY, Seo YK, et al. (2007) Synthesis and catalytic properties of MIL-100 (Fe), an iron (III) carboxylate with large pores. Chem Commun 27: 2820-2822.
- Dhakshinamoorthy A, Alvaro M, Garcia H (2010) Metal-organic frameworks as efficient heterogeneous catalysts for the regioselective ring opening of epoxides. Chem Eur J 16: 8530-8536.
- Dhakshinamoorthy A, Alvaro M, Garcia H (2010) Claisen-schmidt condensation catalyzed by metal‐organic frameworks. Adv Synth Catal 352: 711-717.
- Tran UPN, Le KKA, Phan NTS (2011) Expanding applications of metal-organic frameworks: zeolite imidazolate framework ZIF-8 as an efficient heterogeneous catalyst for the knoevenagel reaction. ACS Catal 1: 120-127.
- Henschel A, Gedrich K, Kraehnert R, Kaskel S (2008) Catalytic properties of MIL-101. Chem Commun 35: 4192-4194.
- Vermoortele F, Ameloot R, Vimont A, Serre C, Vos DD (2011) An amino-modified Zr-terephthalate metal–organic framework as an acid–base catalyst for cross-aldol condensation. Chem Commun 47: 1521-1523.
- Yuan S, Liu TF, Feng DW, Tian J, Wang KC, et al. (2015) [Ti8Zr2O12(COO)16] cluster: an ideal inorganic building unit for photoactive metal-organic frameworks. Chem Sci 6: 3926-3930.
- Chi L, Xu Q, Liang X, Wang J, Su X (2016) Iron‐based metal-organic frameworks as catalysts for visible light‐driven water oxidation. Small 12: 1351-1358.
- Liu XJ, Zhang YH, Chang Z, Li AL, Tian D, et al. (2016) A water-stable metal-organic framework with a double-helical structure for fluorescent sensing. Inorg Chem 55: 7326-7328.
- Yang CX, Ren HB, Yan XP (2013) Fluorescent metal-organic framework mil-53(al) for highly selective and sensitive detection of Fe3+ in aqueous solution. Anal Chem 85: 7441-7446.
- Wang B, Lv XL, Feng D, Xie LH, Zhang J, et al. (2016) Highly stable Zr (IV)-based metal–organic frameworks for the detection and removal of antibiotics and organic explosives in water. J Am Chem Soc 138: 6204-6216.
- Shahat A, Hassan HMA, Azzazy HME (2013) Optical metal-organic framework sensor for selective discrimination of some toxic metal ions in water. Anal Chim Acta 793: 90-98.
- Yassine O, Shekhah O, Assen AH, Belmabkhout Y, Salama KN, et al. (2016) H2S sensors: fumarate‐based fcu‐MOF thin film grown on a capacitive interdigitated electrode. Angew Chem Int Ed 55: 15879-15883.
- Férey G, Draznieks CM, Serre C, Millange F, Dutour J, et al. (2005) A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science 309: 2040-2042.
- Horcajada P, Serre C, Maurin G, Ramsahye NA, Balas F, et al. (2008) Flexible porous metal-organic frameworks for a controlled drug delivery. J Am Chem Soc 130: 6774-6780.
- Zheng H, Zhang Y, Liu L, Wan W, Guo P, et al. (2016) One-pot synthesis of metal-organic frameworks with encapsulated target molecules and their applications for controlled drug delivery. J Am Chem Soc 138(3): 962-968.
- Rojas S, Wheatley PS, Procopio EQ, Gil B, Marszalek B (2013) Metal-organic frameworks as potential multi-carriers of drugs. Cryst Eng Comm 15: 9364-9367.
© 2023 Himadri Acharya. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






