- Submissions

Full Text
Research & Development in Material Science
Study of CoFe2O4@ Calixarene Core-Shell as a Novel Catalyst
Mahsa Aliakbarzadeh Roudsary1, Karim Zare1* and Majid Monajjemi2
1 Department of Chemistry, Islamic Azad University, Iran
2 Department of Chemical engineering, Islamic Azad University, Iran
*Corresponding author: Karim zare, Department of chemistry, Science and research branch, Islamic Azad university, Iran
Submission: May 14, 2018;Published: August 21, 2018

ISSN: 2576-8840 Volume7 Issue5
Abstract
The properties of CoFe2O4@Calix [8] Core-Shell as a novel catalysthasbeen investigatedto compare with well-known catalyst“Fe3O4@Silica”. It has been shown that CoFe2O4 magnetite particle can be use as important catalyst inside the calix ring.In our previous papersgood resultshave been yielded and exhibited about the BnNn ring properties and CoFe2O4@BnNn. In present work it has been shown there is anon-covalent attractionbetween CoFe2O4and calix [8] coated molecules. The Physical-chemistry properties such asenergy densities,potential energy densities,electron densities, ELF, LOL,eta index, elasticity ofelectron density and ECPforCoFe2O4@calix[8] shell have been calculated and simulated in related reactions for those groupsfunctionalized.
Keywords:CoFe2O4; Nano-particles; Electron density; calix [8]; Silica; SiO2
Introduction
CoFe2O4 as the Cobalt ferrite crystallizes in a partially inverse spinel position represented as (Co2x++Fe3+1-x)(Co2+1-xFe3+1+x) O4 where x based on thermal condition. It is ferri-magnetic and exhibits a relatively magnetic hysteresis which distinguishes it from the other of the spinel ferrites. Magnetic measurements on nano-particles of cobalt ferrite dispersed in various solvents of organic compounds and nano-crystalline powders prepared by hydroxideprecipitationhave been investigated earlier. In magnetic fluids, it has been seen that for particlesabove threenano-meters the saturationmagnetization remains constant at about 30emug-1 that is extensively less than the bulk values [1-5].
Magnetic Nan-Oparticles(MNPs) have shown exceptional potential for several biological and clinical applications. However, MNPs might be coated by the biocompatible shells for such applications. The aim of this study is to understand if and how the surfaces charges and coatings can affect the magnetic and electronic properties of Cobalt ferrite crystallizes. The role of the surfaces on the magnetic moments of a magnetic nano particle such as CoFe2O4 is animportant issue, and various effects can contribute for making it deviate from the bulk value, including the charges, the nature of the coating, also the synthetic technique. The electronic properties and ionic distribution of CoFe2O4 NPs were probed by X-ray absorption spectro scopies X-ray-magnetic-circular-dichroism and X-ray-photoemission-spectroscopy-techniques known as the abbreviation XAC, XMCD and XPS respectively. Magnetiteparticlesarealso of interests in medicinal andindustries application such as Magnetic-Resonance-Imaging (MRI) ororganic catalyst and nano-material synthesize [4-8].
The overall magnetic behavior and the hyper-thermic properties were evaluated by magnetometers and molecular modelling measurements, respectively. The results show that all of the investigated CoFe2O4 NPs have high magnetic anisotropyenergy, and the surfaces charges and coating do not influence appreciably their electronic and magnetic properties. In addition, the citrate shell improves the stability of the NPs in aqueous environment, making CoFe2O4 NPs suitable for biomedical applications.Magnetic nano-particle exhibitsseveral unique propertiessuch as super-paramagnetism compared to bulkmaterial andparticularly, are used in the field of biologyand medicines.Magnetic nano-particle has attracted a great deal of researchinterests due to their distinctive properties and special application recently [5-9]. CoFe2O4, is a well-known hard magnetic material with very high cubic magnetocrystalline anisotropy, high coercively and moderate saturation magnetization. These properties make it a promising material for high-density magnetic recording.The most applications require at least a magnetic-particle for dispersing in the non-magnetic matrixes. Thismatrix plays an important role for providing the meaning of particle dispersion for determining a physical property of a composite.
Recently, in thesol-gelsynthesis of CoFe2O4 particles, the gelsare built-up viaphysical and chemical binds between the chemical species.It has been introduced a different sol-gelroutes@ polyacrylamide gel route for preparing CoFe2O4 nano-particle.Due to lack of controls over the specific transformation of anano-particle, obviously super-paramagnetic particle has not been prepared from magnetite, i.e. Magnetite-nano-particle whichgenerallyloses their permanent magnetic properties in the lake of the external magnetic field [6-10]. The complex of metal-CoFe2O4@SiO2-NH2 nano-particlescould be recovered easily from aqueous through magneticseparation and reproducereadily by acid treatment. By this work it has been exhibited the amino-functionalized CoFe2O4@ calix[8] magnetic nano-particles compare to CoFe2O4@SiO2is much more effective as recyclable adsorbent for the removal of heavy and alkali/earth metal ions in water and wastewater treatments. Silica shells chemically is stable and can be rapidly functionalized in the bio-conjugation purposes, in other words is biocompatible therefore CoFe2O4@SiO2 as a silicacoated magnetite composite nano-particles have been synthesized by several groups. Catalysts have a very sensitivetreatment in technology and modern sciences as they increase reaction yield via reducingthe temperature in synthesisof the chemical product.There are two basic types of catalysis, heterogeneous, where the reaction accomplishes on the surfaces and the catalystsare in the solid phase.Homogeneous, where the catalyst is in one phase as reactant.The other important items of these matrixes are to act as a protection for magnetic nano-particlesagainst oxidation or corrosion especiallyin the metallicnano-particles. Among oxide matrixes such as alumina, silica, zeolites, titanic oxides, carbon-based, the silica can be a general suitable material for the matrixes because of inertness ofthe magnetic fields, its non-toxicity and easiness for forming crosslined networks structure [10-17]. The bridge between heterogeneous and homogeneous catalysts can be attained through the CoFe2O4 nanoparticle [17-25]. CoFe2O4exclusivelyis useful and important as the magnetic nano-particle which exhibit strong magnetic-moment and are seldom sustained outside of an external magnetic field. These kinds of nano-particle might be consistsofseveral materials such as nickel, cobalt, iron oxides, and ferritesand also alloys such as platinum/iron. CoFe2O4 MNPs of silica shells catalytic materials have the benefit for increasing surface area which causes for any increased reaction rate. Moreover, nano-particle might permit additional catalytic functionalitybecause of their unique properties. Several catalysis of magnetic nano-structurehas beeninvestigatedup to now,such as preparation of nano-composite materials consist of magnetic-(core) nano-particle which has been coated by various shells of other catalytically active nano-material [20-30].
Other type of catalyststhatare interest for organic compounds involves the using of organic molecules which are enabling forpreservationthe materialsin the end of any reactions for reusing [25-35]. In this work we have investigated the catalysis’s properties of CoFe2O4 nano-particles @calix [8] instead of SiO2 for comparing in chemical synthesizes [36-47]. Recently extensive theoretical and experimental studies have been accomplished on boronnitride- fullerenes for understanding their relative stabilities also size dependence of important physical properties. In our previous works [48-61], it has been exhibited thesystemstabilities, NMR data, electronic properties, and chemical phenomenon.
Background & Methodology
Magnetic particles are suitable for aqueous transition and heavy metals due to their unique advantages of quick separation and their high surface areaunder external magnetic fields [62-65]. The surface modification, adsorption affinity, including covalentbinding and physical coating, has often been exploredforenabling specific complexationfor further facilitates [66-70]. Recently it have been exhibited which the amino-functionalized molecules demonstrated outstanding abilities for removing a wide variety of transition heavy metalions [71-76]. Although CoFe2O4@SiO2has recently been investigated for potential biomedical applications [77-81], there is no work about the CoFe2O4@calix [8].
In this studywith the theoretical approaches magnetic an adsorbent has been developed via covalently grafting amino groupsover the surfaces of CoFe2O4@calix[8] nano-particles. Part of the systems including CoFe2O4@calix [8] nano-particles has been simulated with QM/MM methods and the investigation carried out by the Monte Carlo calculations.In this study, various force fields are donevia“Amber” and OPLSfor comparing the calculated energy of the CoFe2O4@ calix [8] nano-particles. Furthermore, a Hyper-Chem professional release-7.01program is used for any further calculations.Thedensity functional method is used for the high levelwhile the semi-empirical (pm6) with pseudo=Lanl2 and Pm3MM for both of them respectively.Some accurate studies have indicated that in-accuracy of the low range exchange energies goes tothe large systematic errors for the prediction of molecular properties.
Geometries optimization and electronic calculations have been accomplished using the m06 functional of DFT. These approachessare based on solution of the Kohn & Sham [82] in the plane-waves sets with projector-augmented-pseudo-potentials. The Perdew & Burke [83] exchange-correlation and generalizedgradient- approximation GGA are also used for non-bonding calculation.
The charge transferring and electrostatics potentialsderived charges were also estimated using the Merz & Kollman [84]; [85,86]. The charges calculation methods based on MESP or molecular-electrostatics-potentials fitting are not wellsuited for the larger systems whereas several of the inner-most points are located far away from the centers at which the MESP are computed. In that position, variation of the inner-most atomic charges would not be towards the changing of the MESP outside of the molecules [87,88]. The interaction energies or adsorbents energies between metals and CoFe2O4@calix[8]catalyst were done according to the equation as follows:
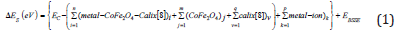
That S ΔE is the adsorbents energies. The electron-localizationfunction or ELF, localized-orbital-locator or LOL [89-91], electron density of the Gradient-norm & Laplacian, values of orbitals wave-functions, electron spin densities, electrostatic-potentials from nuclear-atomic-charges,the exchange-correlation density, as well astotal electrostatic potentials ESP), correlation-holes and correlation-factors, and the average local ionization energies using the Multi-functional-Wave-function analyzer have also been calculated in this study [89-91].
Density Electron Approach for Interaction between Mnps and Calix [8]
The kinetic energies densitiesare not defined individually, since the expected values of theoperators:

Can be estimated by integrating kinetic energy densities from those alternatives definitions. One of the usual used definitionsis as follows:
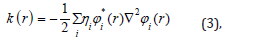
Thelocal kinetic energies given below guarantee [89-91] hence the physical dataare more commonly used. The Lagrangian of kinetic energies densities, G(r)97are also known as positive definite kinetic energy densities.
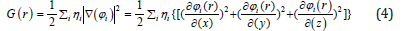
K(r) and G(r) are directly related by Laplacian of electron density
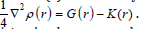
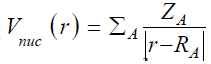
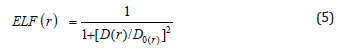
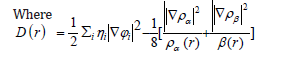
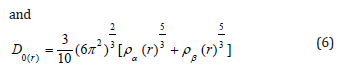
for close-shell system, since
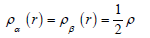
Dand D0 terms can be simplified as
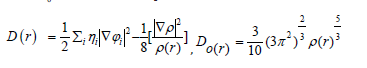
In which the kinetic energies terms in D(r) is replaced by Kirzhnits types second-order gradients expansion, which are
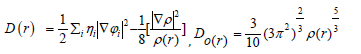
So that ELF is totally independent from the wave-function, and then can be used foranalyzing electron densities from X-ray diffraction data.Localized orbital locator orLOLis another item for locating high localization regions likewise ELF, which explained by Lu T [91].
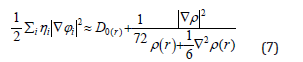
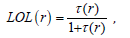
for spin-polarized system and close-shell system are defined in the same way as in ELF. LOL have similar approaches compared to ELF.Notice that evaluating ESP is much more time-consuming than evaluating other functions. The ESP evaluated under default value is accurate enough in general cases. Reduced density gradient (RDG) RDG are a pair of very important functions for revealing weak interaction regionfor detail. RDG is defined as
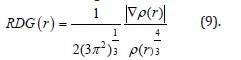
Fortunately, it is found that weak interaction analysis under pro-molecular density is still reasonable. Pro-molecular density is simply constructed by superposing electron densities of free-state atoms and hence can be evaluated extremely rapidly.
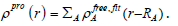
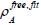
Result and Discussion
Table 1:(a) All Electron Densities of non-bonded interactions for CoFe2O4@Silica shell, (b) CoFe2O4@calix[8] shell and(c) CoFe2O4 (isolate).
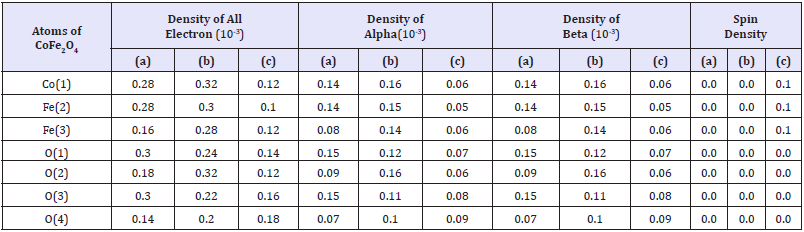
Figure 1:The non-bonded interaction between CoFe2O4 and Calix [8] shell.
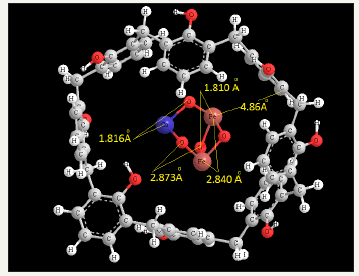
Figure 2:The optimized of CoFe2O4.
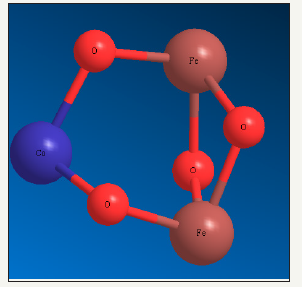
Figure 3:Color field map of electro density including molybdenum & CoFe2O4 inside of Calix [8].
Figure 4:Contour line map ofCoFe2O4-Calix [8]for LOL.

Figure 5:ELF versus Position for CoFe2O4@calix[8] & Mo.
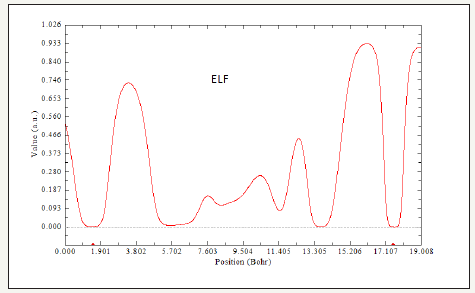
Figure 6:Pick Position for CoFe2O4@calix[8] Mo & V (+5).
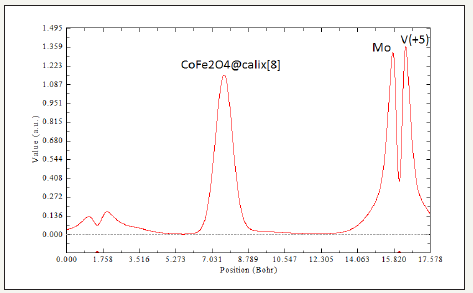
Thisworkbasically focuses on the magnetic properties of CoFe2O4 in the non-bonded systems with calix[8] surfaces including “CoFe2O4@calix [8]”. The CoFe2O4@calix[8]nano-adsorbent shown high adsorption affinities for aqueousion metalsspecially for cation of vanadium and molybdenum and the amino-CoFe2O4@ SiO2exhibited high adsorbent forvanadium and molybdenum ions, resulting from complexation of the metals ion with the surface amino groups.The metalloaded CoFe2O4@calix[8] nano-particles couldM be recovered easily from aqueous solution bymagneticseparation. The data have shown in Figure 1-7 and Table 1. As it is indicated in Table 1, LOL is low and constant for Both CoFe2O4@silica, CoFe2O4@ calix[8] and CoFe2O4@calix [8]. ELF has a similar expression as LOL. The non-bonded interactions areshown in Figure 1-7.The electrical properties can be obtained from changes in the nonbonded interactions. Potential energy densities, ELF, LOL,electron densities, energy densities,eta index and ECP are shown Table 1. The results of ELF and LOL indicate that the surfaces of silica and calix [8] are suitable to attach in aromatic and organic compounds in any scale from nano to micro or medium.
Figure 7:Density of state for energy absorption of Molybdenum and Vanadium in CoFe2@Calix[8].
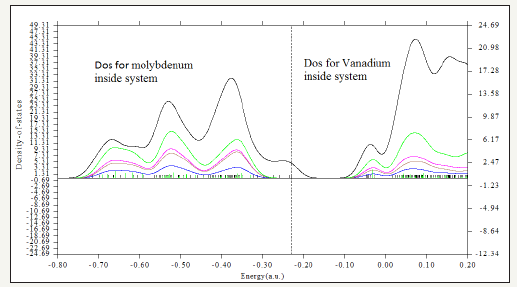
The interaction energy between two sides of CoFe2O4-silica and CoFe2O4-calix [8] are also calculated Table 1. This problem will removed by replacing the calix[8] instead of SiO2 as a shell of CoFe2O4. In addition, functional groups on the coating layer chemically adhering to the MNPs are also assailable to acid treatment.In contrast of SiO2 rings which is stable under acidic conditions the calix [8]rings is independent from acidic situations and hence for calix[8] comparing to SiO2, the functions is not needed as an ideal shell composite to protect the inner magnetite core. Although CoFe2O4@SiO2 or Silica-coated core–shell magnetite nanoparticles have recently been investigated for potentiallbiomedical applications, by this work we exhibit the calix [8] is much useful for removing the earth/alkali metal from the aqueous solution Table 1. Relative adsorption energies of fourtransition metalions such as vanadium and molybdenum on CoFe2O4@calix[8]have investigated.
Conclusion
We think that calix[8] capabilities of magnetic substrates should be explored in the near future.Another interesting development is using the calix[8] on magnetic-nano-particle enables effective removal of transition metals such as vanadium and molybdenum metals based on catalysts forms important pharmaceutical products in the drug nanotechnology. Our Calculations indicate that the calix[8]is suitable surfaces for CoFe2O4such silica surfaces for removal metal ions such as vanadium cations and molybdenum.
References
- Massart R (1981) Preparation of aqueous magnetic liquids in alkaline and acidic media IEEE transactions on magnetics 17(2): 1247-1248.
- Valter S, Richard TO, Rao KV (2010) Real-time monitoring of the evolution of magnetism during precipitation of superparamagnetic nanoparticles for bioscience applications. J Mater Chem 20: 4168-4175.
- Shi M, Zuo RZ, Xu YD, Jiang YZ, Yu GY, et al. (2012) Preparation and characterization of CoFe2O4 powders and films via the sol-gel method. J Alloy Compd 512: 165-170.
- Cui HT, Jia YY, Ren WZ, Wang WH (2010) Facile and ultra-large-scale synthesis of nearly monodispersed CoFe2O4 nanoparticles by a low temperature sol-gel route. Journal of Sol-Gel Science and Technology 55(1): 36-40.
- Burianova S, Vejpravova JP, Holec P, Plocek J, Niznansky D (2011) Surface spin effects in La-doped CoFe2O4 nanoparticles prepared by microemulsion route. J ApplPhys110: 073902.
- Haneda K, Morrish AH (1998)J Appl Phys 63(8): 42-58.
- MoumenN, Veillet P, Pileni MP (1995) Controlled preparation of nanosize cobalt ferrite magnetic particles. Journal of Magnetism and Magnetic Materials 49(1-2):67-71.
- Blaskov V, Petkov V, Rusanov V, Martinez LM, Martinez B, et al. (1996) Magnetic properties of nanophase CoFe2O4 particles. Journal of Magnetism and Magnetic Materials162(2-3): 331-337.
- Pillai V, Shah DO (1996) Synthesis of high-coercivity cobalt ferrite particles using water-in-oil micro-emulsions. Journal of Magnetism and Magnetic Materials 163(1-2): 243-248.
- Davis KJ, Well S, Upadhyay SV, Charles SW, OGrady KM, et al. (1995) The observation of multi-axial anisotropy in ultrafine cobalt ferrite particles used in magnetic fluids. Journal of Magnetism and Magnetic Materials 149(1-2): 14-18.
- Ming M, Yu Z, Zhir G, Ning GU (2013) Facile synthesis of ultrathin magnetic iron oxide nanoplates by Schikorr reaction. Nanoscale Research Letters 8(1): 16.
- Zhu ZG, Li XY, Zhao QD, Shi Y, Li H, et al. (2011) Surface photovoltage properties and photocatalytic activities of nanocrystalline CoFe2O4 particles with porous superstructure fabricated by a modified chemical coprecipitation method. Journal of Nanoparticle Research 13(5): 2147- 2155.
- Zhang Y, Liu Y, Yang Z, Xiong R, Shi J (2011) Synthesis of CoFe2O4 nanoparticles with tunable magnetism by the modified hydrothermal method. Journal of Nanoparticle Research 13: 4557.
- Peng JH, Hojamberdiev M, Xu YH, Cao BW, Wang J, et al. (2011) Hydrothermal synthesis and magnetic properties of gadolinium-doped CoFe2O4 nanoparticles. Journal of Magnetism and Magnetic Materials 323: 133-137.
- Yanase A, Siratori K (1984) Band structure in the high temperature phase of Fe3O4. J Phys Soc Jpn53: 312-317.
- Pe´nicaud M, Siberchicot B, Sommers CB, Kubler J (1992) Calculated electronic band structure and magnetic moments of ferrites. Journal of Magnetism and Magnetic Materials 103(1-2): 212-220.
- Babes L, Denizot B, Tanguy G, Le Jeune JJ, Jallet P (1999) Synthesis of iron oxide nanoparticles used as MRI contrast agents: A parametric study. Journal of Colloid and Interface Science 212(2): 474-482.
- Berry C, Curtis ASG (2003) Functionalisation of magnetic nanoparticles for applications in biomedicine. Journal of Physics D: Applied Physics 36(13): R198-R206.
- Ruuge EK, Rusetski AN (1993) Magnetic fluids as drug carriers: Targeted transport of drugs by a magnetic field. Journal of Magnetism and Magnetic Materials 122(1-3): 335-339.
- Pope NM, Alsop RC, Chang YA, Smith AK (1994) J Biomed Mater Res 2: 449-457.
- Tago T, Hatsuta T, Miyajima K, Kishida M, Tashiro S, et al. (2002) Novel synthesis of silica-coated ferrite nanoparticles prepared using waterin- oil microemulsion. Journal of the American Ceramic Society 85(9): 2188-2194.
- Lei Z, Li YL, Wei XY (2008) A facile two-step modifying process for preparation of poly(SStNa)-grafted Fe3O4/SiO2 particles. Journal of Solid State Chemistry 181(3): 480-486.
- Huang C, Bin H(2008)Silica-coated magnetic nanoparticles modified with γ-mercaptopropyltrimethoxysilane for fast and selective solid phase extraction of trace amounts of Cd, Cu, Hg, and Pb in environmental and biological samples prior to their determination by inductively coupled plasma mass spectrometry. Spectrochim Acta Part B: Atomic Spectroscopy 63(3): 437-444.
- Philipse AP (2011) Heterogeneous catalysis: On bathroom mirrors and boiling stones.J Chem Educ 88(1): 59-62.
- Sheldon RA, Downing RS (1999) Heterogeneous catalytic transformations for environmentally friendly production.Applied Catalysis A: General 189(2): 163-183.
- Hamilton DJ (2003) Homogeneous catalysis-New approaches to catalyst separation, recovery, and recycling. Science 299(5613): 1702-1706.
- Cornils B, Herrmann WA (2003) Concepts in homogeneous catalysis: the industrial view. Journal of Catalysis 216(1-2): 23-31.
- Johnson BG (2003) Nanoparticles in catalysis. Topics in Catalysis 24(1- 4): 147-159.
- Laurent S, Forge D, Port M, Roch A, Robic C, et al. (2008) Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev 108(6): 2064-2110.
- Kodama RH (1999) Magnetic nano-particles. Journal of Magnetism and Magnetic Materials 200(1-3): 359-372.
- Chang LL, Erathodiyil N, Ying JY (2012) Nanostructured catalysts for organic transformations. Acc Chem Res 46(8): 1825-1837.
- Fujishima A, Zhang X, Tryk DA (2008) TiO2 photocatalysis and related surface phenomena Surface. Science Reports 63(12): 515-582.
- Wang X, Gaiano SM, Bridges T, Montell D (2008) ProtocExch28.
- Wei S, Wang Q, Zhu J, Sun, L, Lin, et al. (2011) Multifunctional composite core-shell nanoparticles.Nanoscale 3(11): 4474-4502.
- Yoon TJ, Lee W, Oh YS, Lee JK (2003) Magnetic nanoparticles as a catalyst vehicle for simple and easy recycling New. Journal Chemisty 27(2): 227- 229.
- Yu Zh, Hu ML, Zhang CX, Sun LZ, Zhong JJ (2011) Tunneling magneto resistance of bilayer hexagonal boron nitride and its linear response to external uniaxial strain. J Phys Chem C 115(16):8260-8264.
- Seifert G, Flower PW, Mitchell D, Porezag D, Frauenheim T (1997) Boron-nitrogen analogues of the fullerenes: electronic and structural properties. Chemical Physics Letters 268(5-6): 352-358.
- Alexandre SS, Chacham H, Nunes RW (1999) Stability, geometry, and electronic structure of the boron nitride B36N36 fullerene. Appl Phys Lett 75: 61.
- Locke IW, Darwish AD, Kroto HW, Prassides K, Taylor R, et al. (1994) Phosphorus/buckminsterfullerene intercalation compounds, C60(P4)2. Chemical Physics Letters 225(1-3): 186-190.
- Behrman EC, Foehrweiser RK, Myers JR, French BR, Zandler ME (1994) Possibility of stable spheroid molecules of ZnO. Phys Rev A 49: R1543.
- Kaxiras E, Jackson K, Pederson MR (1994) Theoretical study of passivated small fullerenes C24X4 (XN,P,As) and their isoelectronic equivalents (BN)12X4. Chemical Physics Letters 225(4-6): 448-453.
- Zhao JX, Ding YH (2008) Theoretical study of ni adsorption on singlewalled boron nitride nano-tubes with intrinsic defects. J Phys Chem C 112(15): 5778-5783.
- Bagheri S, Moosavi MS, Moradiyeh N, Zakeri M, Attarikhasraghi N, et al. (2015) Molecules 20: 21636-21657.
- Strout DL (2004) Fullerene-like cages versus alternant cages: isomer stability of B13N13, B14N14, and B16N16Chemical. Physics Letters 383(1-2): 95-98.
- Zope RR, Dunlap BI (2004) Are hemispherical caps of boron-nitride nanotubes possible? Chemical Physics Letters 386(4-6): 403-407.
- Zope RR, Baruah T, Pederson MR, Dunlap BI (2004) Electronic structure, vibrational stability, infrared, and Raman spectra of B24N24 cages. Chemical Physics Letters 393(4-6): 300-304.
- Zheng JW, Zheng LP, Wu P (2010) Theoretical study of Li, Si and Sn adsorption on single-walled boron nitride nanotubes. J Phys Chem C 114(13): 5792-5797.
- Monajjemi M, Lee VS, Khaleghian M, Honarparvar B, Mollaamin F (2010) Theoretical description of electromagnetic nonbonded interactions of radical, cationic, and anionic NH2BHNBHNH2 inside of the B18N18nanoring. J Phys Chem C 114(36): 15315-15330.
- Monajjemi M (2017) Liquid-phase exfoliation (LPE) of graphite towards graphene: An ab initio study. Journal of Molecular Liquids 230: 461-472.
- Monajjemi M (2012) Quantum investigation of non-bonded interaction between the B15N15 ring and BH2NBH2(radical, cation, anion) systems: a nanomolecularmotor. Structural Chemistry 23(2): 551-580.
- Monajjemi M, Boggs JE (2013) A new generation of BnNn rings as a supplement to boron nitride tubes and cages. J Phys Chem A117(7): 1670-1684.
- Monajjemi M, Khaleghian M (2011) EPR Study of electronic structure of [CoF6]3−and B18N18nano ring field effects on octahedral complex. Journal of Cluster Science 22(4): 673-692.
- Monajjemi M, Wayne R, Boggs JE (2014) NMR contour maps as a new parameter of carboxyl’s OH groups in amino acids recognition: A reason of tRNA–amino acid conjugation. Chemical Physics 433: 1-11.
- Monajjemi M, Falahati M, Mollaamin F (2013) Computational investigation on alcohol nanosensors in combination with carbon nanotube: a Monte Carlo and ab initio simulation. Ionics 19(1): 155-164.
- Monajjemi M, Mollaamin F (2012) Molecular modeling study of drug- DNA combined to single walled carbon nanotube. Journal of Cluster Science23(2): 259-272.
- Mollaamin F, Monajjemi M (2012) DFT outlook of solvent effect on function of nano bioorganic drugs. Physics and Chemistry of Liquids 50(5): 596-604.
- Monajjemi M, Khosravi M, Honarparvar B, Mollaamin F (2011) Substituent and solvent effects on the structural bioactivity and anticancer characteristic of catechin as a bioactive constituent of green tea. International Journal of Quantum Chemistry 111(12): 2771-2777.
- Monajjemi M (2015) Non-covalent attraction of B2N(−,0)and repulsion of B2N(+)in the BnNn ring: A quantum rotatory due to an external field. Theoretical Chemistry Accounts 134:77.
- Monajjemi M (2014) Metal-doped graphene layers composed with boron nitride-graphene as an insulator: A nano-capacitor Journal of Molecular Modeling 20: 2507.
- Monajjemi M, Khaleghian M, Mollaamin F (2010) Theoretical study of the intermolecular potential energy and second virial coefficient in the mixtures of CH4 and Kr gases: a comparison with experimental data. Molecular Simulation 36(11): 865-870.
- Monajjemi M (2015)Cell membrane causes the lipid bilayers to behave as variable capacitors: A resonance with self-induction of helical proteins. Biophysical Chemistry207: 114-127.
- Jalilian H, Monajjemi M (2015) Capacitor simulation including of X-doped graphene (X=Li, Be, B) as two electrodes and (h-BN)m (m=1-4) as the insulator. Japanese Journal of Applied Physics54(8): 085101.
- Shin S, Jang J (2007) Thiol containing polymer encapsulated magnetic nanoparticles as reusable and efficiently separable adsorbent for heavy metal ions. Chem Commun 41: 4230-4232.
- Oliveira LCA, Petkowicz DI, Smaniotto A, Pergher SBC (2004) Magnetic zeolites: a new adsorbent for removal of metallic contaminants from water. Water Res 38(17): 3699-3704.
- Hu J, Chen G, Lo IMC (2005) Removal and recovery of Cr (VI) from wastewater by maghemite nanoparticles. Water Res 39(18): 4528-4536.
- Yavuz CT, Mayo JT, Yu WW, Prakash A, Falkner JC, et al. (2006) Lowfield magnetic separation of monodisperse Fe3O4nanocrystals. Science 314(5801): 964-967
- Hai B, Wu J, Chen X, Protasiewicz JD, Scherson DA (2005) Metal-Ion adsorption on carboxyl-bearing self-assembled monolayers covalently bound to magnetic nanoparticles. Langmuir 21(7): 3104-3105.
- Hu J, Lo MC, Chen GH (2007) Performance and mechanism of chromate (VI) adsorption by δ-FeOOH-coated maghemite (γ-Fe2O3) nanoparticles. Sep Purif Technol 58(1): 76-82.
- Chang YC, Chen DH (2005) Preparation and adsorption properties of monodisperse chitosan-bound Fe3O4 magnetic nanoparticles for removal of Cu(II) ions. J Colloid Interface Sci 283(2): 446-451.
- Liu JF, Zhao ZS, Jiang GB (2008) Coating Fe3O4 magnetic nano-particles with humic acid for high efficient removal of heavy metals in water. Environ Sci Technol 42(18): 6949-6954.
- Yantasee W, Warner CL, Sangvanich T, Addleman RS, Carter TG, et al. (2007) Removal of heavy metals from aqueous systems with thiol functionalized superparamagnetic nano-particles. Environ Sci Technol 41(14): 5114-5119.
- Kumar M, Rathore DPS, Singh AK (2000) Amberlite XAD-2 functionalized with o-aminophenol: synthesis and applications as extractant for copper(II), cobalt(II), cadmium(II), nickel(II), zinc(II) and lead(II). Talanta 51(6):1187-1196.
- Lam KF, Yeung KL, McKay G (2007) Efficient approach for Cd2+ and Ni2+removal and recovery using mesoporous adsorbent with tunable selectivity. Environ Sci Technol 41(9): 3329-3334.
- Li J, Miao X, Hao Y, Zhao J, Sun X, et al. (2008) Synthesis, aminofunctionalization of mesoporous silica and its adsorption of Cr(VI). J Colloid Interface Sci 318(2): 309-314.
- Liu Q, Xu Z, Finch JA, Egerton R (1998) A novel two-step silica-coating process for engineering magnetic nano-composites. Chem Mater 10(12): 3936-3940.
- Lu CW, Hung Y, Hsiao JK, Yao M, Chung TH, et al. (2007) Bifunctional magnetic silica nanoparticles for highly efficient human stem cell labeling. Nano Lett 7(1): 149-154.
- Ashtari P, He XX, Wang K, Gong P (2005) An efficient method for recovery of target DNA based on amino-modified silica-coated magnetic nanoparticles. Talanta 67(3):548-554.
- Zhao Y, TruhlarDG (2008) The Mo6 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four Mo6-class functionals and 12 others functional. Theor Chem Account 120(1-3):215-241.
- Zhao, Y, Truhlar, DG (2008) Density functionals with broad applicability in chemistry. Accounts of Chemical Research 41(2): 157-167.
- Grimme S (2006) Seemingly simple stereo electronic effects in alkane isomers and the implications for kohn-sham density functional theory. ChemInt Ed (45): 4460-4464.
- Schreiner PR, Fokin AA, Pascal RA, de Meijere A (2006) Many density functional theory approaches fail to give reliable large hydrocarbon isomer energy differences. Org Lett 8(17): 3635-3638.
- Kohn W, Sham LJ (1965) Self-consistent equations including exchange and correlation effects. Phys Rev (140)A: 1133-1138.
- Perdew JP, Burke K (1996) Generalized gradient approximation made simple. Phys Rev Lett (77): 3865-3868.
- Besler BH, Merz KM, Kollman PA (1990) Atomic charges derived from semiempirical methods. J comp Chem 11(4): 431-439.
- Brneman CM, Wiberg KB (1990) Determining atom‐centered monopoles from molecular electrostatic potentials: The need for high sampling density in formamide conformational analysis. J Comp Chem 11(3): 361- 373.
- Chirlian LE, Francl MM (1987) Atomic charges derived from electrostatic potentials: A detailed study. J Comp Chem 8(6): 894-905.
- Martin F, Zipse H (2005) Charge distribution in the water molecule-A comparison of methods. J Comp Chem 26(1): 97-105./a>
- Balderchi A, Baroni S, Resta R (1998) Evolution of the dendritic instability in solidifying succinonitrile. Phys Rev Lett (61): 173.
- Lu T, Chen F (2011)Calculation of molecular orbital composition. Acta Chim Sinica 69: 2393-2406.
- Lu T, Chen F (2012) Quantitative analysis of molecular surface based on improved marching tetrahedral algorithm. J Mol Graph Model 38: 314- 323.
- Lu T, Chen F (2012) Multiwfn: A multifunctional wave function analyzer. J Comp Chem 33(5): 580-592.
© 2018 Karim Z. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






