- Submissions

Full Text
Research & Development in Material Science
Brief on Catalytic Reactions to Maximize Production and Minimize Pollution (MPMP)
SSEH Elnashaie*
Department of Chemical and Biological Engineering, University of British Columbia, Canada
*Corresponding author: SSEH Elnashaie, Department of Chemical and Biological Engineering, University of British Columbia, Vancouver, Canada
Submission: February 09, 2018; Published: March 19, 2018

Volume 4 Issue 3 March 2018
Opinion
One of the main techniques for pollution control and achieving green technology is to achieve MPMP. Almost all catalytic reactions in the Petroleum refining and petrochemical industry are reversible and therefore their conversion is limited by the thermodynamic equilibrium. This conservative limitation can be broken by using selective membranes to remove one of the products. In this couple of pages editorial this revolutionary concept leading to MPMP is used for the dehydrogenation reaction where the selective membranes are used for the perm-selective removal of hydrogen [1-3]. These membranes have 100% selectivity for the removal of hydrogen. Most efficient configuration is when in the other side of the membrane is a hydrogenation reaction and the flows in the two sides of the membrane are counter-current. Such MPMP for catalytic reactors is essential part of Sustainable Development Engineering (SDE) which is an important subsystem of Sustainable Development (SD). The removal of one, or more, of the products relaxes this limitation and increases the conversion of the reaction; this relaxation increases as the removal of the product(s) is increased. The optimal utilization of this approach leads to MPMP approaching green technology and is a part of SDE. This editorial is concentrating on the removal of hydrogen from a dehydrogenation reaction, mainly ethyl-benzene to styrene [1-3], using hydrogen perm-selective membranes. The rate of hydrogen removal from the reaction side depends upon the type of the membrane and also the hydrogen driving force between the two sides of the membranes. This driving force increases when there is a hydrogenation reaction in the other side of the membrane. In this editorial a hydrogenation reaction of nitrobenzene to aniline is taking place on the other side of the membranes.
Figure 1: Schematic diagram showing integrated reactor configuration.
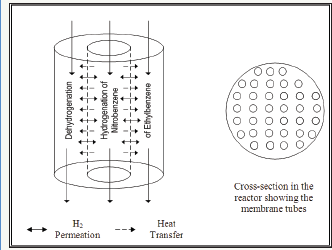
Figure 2: Hydrogen profiles for different configurations/ sides.
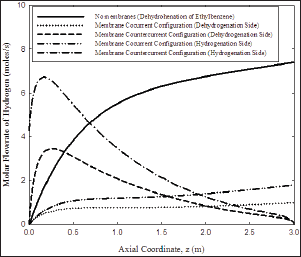
Figure 1 shows a schematic diagram for this novel integrated membrane reactor. Figure 2 shows the hydrogen profiles for both co-current and counter-current configurations. For counter-current case, feed is from the right for the hydrogenation compartment; otherwise all feeds are from the left. The counter-current is obviously more efficient.
References
- She Y, Han J, Ma YH (2001) Palladium membrane reactor for the dehydrogenation of ethylbenzene to styrene. Catal Today 67(1-3): 4353.
- Hermann Ch, Quicker P, Dittmeyer R (1997) Mathematical simulation of catalytic dehydrogenation of ethylbenzene to styrene in a composite palladium membrane reactor. J Membr Sci 136(1-2): 161-172.
- Abashar MEE (2003) Coupling of ethylbenzene dehydrogenation and benzene hydrogenation reactions in fixed bed catalytic reactors. Chem Eng Pro 43: 1195-1202.
© 2018 SSEH Elnashaie. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






