- Submissions

Full Text
Research & Development in Material Science
Role of Diamond Nanoparticles in Ni Based Protective Coatings
Sankaran Murugesan, Othon R. Monteiro, Radhika Suresh and Valery N Khabashesku*
Department of Nanotechnology, Baker Hughes a GE company, USA
*Corresponding author: Khabashesku VN, Department of Nanotechnology, Center for Technology Innovation, Baker Hughes a GE Company, 14990 Yorktown Plaza Drive, Houston, TX, 77040 USA
Submission: November 20, 2017;Published: December 15, 2017

ISSN : 2576-8840Volume2 Issue4
Abstract
Protective coatings are important for mitigation of the corrosion and erosive wear of equipment components and infrastructure in oil and gas industry. Ni-based metal-matrix nanocomposite (MMnC) coatings incorporated with diamond nanoparticles offer an interesting alternative to hard chromium and conventional Ni coatings. It is expected that components manufactured from inexpensive base materials coated with MMnCs should have longer service life, which translates into lower operating costs. In the present work, two different Ni-based MMnCs filled with nanodiamond particles were developed through Ni-P electroless and Ni-B electroplating deposition. The role of co-deposited diamond nanoparticles in these Ni-based MMnCs has been assessed by following the relationship between hardness and corrosion potential and microstructure and particle size of the coatings. The results presented in this paper demonstrate that Ni-based MMnC coatings outperform conventional electroless Ni-P and electroplated Ni-B coatings and validate their ability to provide simultaneous corrosion and erosion protection for oil and gas tools and components.
Introduction
Maintenance and repair expenses in the oil and gas industry caused by wear, erosion and corrosion are very large. To a great extent, corrosion phenomena experienced in oil and gas systems are based on aqueous corrosion caused by soluble corrosive gases, which include carbon dioxide, hydrogen sulfide and oxygen. Corrosion is additionally accompanied by abrasive wear, resulting from interaction between moving parts or erosive wear caused by interaction with particulate-loaded flowing fluids. Erosive wear can be mitigated with the use of hard and tough surface coatings [1]. Generally, chromium coatings have been utilized for this purpose in the industry. However, the increasing pressure to eliminate hard chromium in oil and gas applications due to environmental concerns related to hexavalent chromium has fueled the need for substitutes with comparable economics and performance. Nickel (Ni) presents a viable alternative coating material that has been widely used in industrial applications over the past five decades because of its unique characteristics that improve resistance to corrosion, abrasion and wear. Performance of Ni-based coatings can be further advanced with the development of MMnCs.
The composite coatings described here are formed by simultaneous deposition of the nanoparticles particles and nickel on the growth surface with the envelopment of the former by the depositing Ni ions. In the case of composite coatings, the particle content, size, and in some cases the orientation affect the ultimate properties of the coatings [2]. MMnCs are reinforced with the particles added into the metal matrix that results in remarkable improvement of mechanical properties. Several different types of particles, such as oxides (Al2O3, Y2O3), nitrides (Si3N4, AlN), carbides (TiC, SiC), hydrides (TiH2) and borides (TiB2), have been employed as reinforcement additives [3] with carborundum and alumina becoming the most common ceramic reinforcements for MMnCs. Earlier efforts on preparing Ni-diamond composite coatings with different plating conditions and bath compositions have shown the benefits of composite coatings over monolithic metallic coatings [47]. Furthermore, we have shown that the Ni-P and Ni-B coatings with the added particle provide an overall improvement towards erosion and corrosion resistance [8-10].
In general, the electroplated and the electroless deposited Ni-P and Ni-B coatings are amorphous and may contain only a very small nanocrystalline domain ranging from 2 to 20nm. As deposited, these coatings are hard and relatively brittle. Corrosion resistance of Ni-P is superior to Ni-B. Following the deposition, a heat treatment at temperatures between 250 oC and 400 oC precipitates Ni3P or Ni3B crystals out of the matrix. This heat treatment improves the mechanical properties and corrosion resistance of the coatings [11]. These coatings are typically produced with the P or B concentrations varying from 2 to 14wt.%, where the exact content determines their properties. Electroless deposition is usually performed at 80 to 90 oC and at high pH conditions. The typical fabrication of these coatings uses an electroless process. Conventional electroplating can provide an alternative route for simpler geometries. Electroplating is typically conducted at lower temperatures, around 50 oC, and for Ni-B coatings traditionally uses boric acid, boron hydrides or carboranes as boron sources. Recently, a number of publications have suggested the use of trimethylamine or dimethylamine borane [12,13]. The absence of strong reducing agents in electroplating bath prevents the self- catalytic precipitation of Ni particles from solution [14] and makes this process inherently more stable than electroless deposition. Previously, we stated that with use of non-traditional boron source, which enables higher B-contents in the matrix, superior hardness and resistance to corrosion can be achieved [9]. In this paper we describe the impact that boron, phosphorus and nanodiamond, incorporated in the matrix, have on growth, microstructure, corrosion and mechanical properties of this new class of metal matrix nanocomposite coatings.
Experimental Section
Ni-P and Ni-P-diamond composite coatings were produced by electroless deposition on tool-grade 4140 steel coupons and then heat treated at 400 oC for 1 hour in air. Electroless Ni-P coatings were prepared with the concentration of phosphorus as high as approximately 10wt.%. The high-P monolithic and nanocomposite films were prepared in a mechanically stirred electroless bath at a pH of 9 and at 85 oC. During the deposition of nanocomposite coatings, diamond particles with an average particle size of 100 nm were added to the bath and mechanically stirred throughout the process. Coatings were prepared by using a bath solution with 2.5wt.% diamond concentration. Prior to deposition, the 4140 tool steel coupons were polished down to 600 meshes and washed in a caustic soap at room temperature, followed by rinse in ethanol and short (10sec) activation in 10% HCl solution. A final rinse in de-ionized water was conducted immediately prior to the start of the coating.
Electroplating was carried out in a conventional Watts bath modified with the addition of 1wt.% trimethylamine borane as the source of boron. Depositions were carried out at 0.1 A/dm2 current density for 1 hour at 50oC. The pH of the solution was adjusted to a value of 3.5 using 30% HCl. In the electrolyte bath other additives used to modify the film microstructure were sodium dodecyl sulfonate (SDS) and saccharine. Ni-B-nanodiamond composite coatings with the average particle size of 100nm added to the Watts bath prior to the insertion of the cathode. The bath solution was mechanically stirred, and the weight content of the nanoparticles in the bath remained unchanged during the deposition.
The morphology of the coatings was characterized by scanning electron microscope (SEM) (model JSM-7800). Microhardness measurements were conducted with a Knoop microindenter at a load of 50g. The values reported in this paper are the averages of five measurements.
Cyclic potentiodynamic polarization measurements were performed to determine relative susceptibility to localized corrosion in 3.5% NaCl water solution (chloride environment) at room temperature according to ASTM (G 61-86). This is the standard test used to find the localized corrosion susceptibility of iron-, nickel- or cobalt-based alloys; its application was extended to evaluate the performance of the protective coatings.
Results and Discussion
Figure 1: SEM images of the surfaces of coatings.
1a: Electroless Ni-P;
1b: Electroplated Ni-B;
1c: Ni-P-nanodiamond with average diamond particles size of 70nm;
1d: Ni-B-nanodiamond with average diamond particle size of 100nm.

Figure 1a & 1b shows the SEM images of electroless Ni-P and electroplated Ni-B coatings having the cauliflower-like structure, which is typical of films produced under conditions of limited atomic mobility. Incorporation of the nanoparticles changes the morphology of the coating, leading to significantly finer domes, as shown in Figure 1c & 1d. Ashassi-Sorkhabi & Es'haghi [15] have postulated that incorporation of nanoparticles during deposition of composite coatings disrupts the boundaries of the Ni-P/Ni-B clusters, breaking down the otherwise "columnar" structure, resulting in a morphology with finer dome size. In both cases the coatings studied here were 50|im thick. The phosphorous and boron concentration determined by EDS was approximately 10wt.% for P in Ni-P coatings and 8wt.% boron in Ni-B coatings.
The effect of hard particles on the erosive wear of composite materials is often described by the following relationship (1):
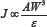
Where J is the erosive wear rate, W is the flow velocity, A is the particle shape and s is the erosion resistance that is directly related to the ratio of the hardness of the impinging particle to the hardness of the substrate. According to Equation (1) the erosive wear is inversely related to the hardness of the eroded material. Several authors assumed that the hardness of a composite material can be described by the direct rule of mixtures, i.e., the weighted average of the hardness of the different phases [16], although this is not proven to hold in all cases. The presence of the dispersed phase in electroless or electroplated coatings affects the microstructure and the hardness of the matrix phase in Ni-based coatings [8-10]. Moreover, the adhesion between the particles and the matrix plays an important role in determining the hardness as well as other mechanical properties of the composite material.
Figure 2: Knoop Hardness of coatings, electroless high Ni-P content monolithic and with nanodiamond, measured before and after heat treatment at 400 °C for 1h, and Ni-B coatings prepared from a Watts bath with TMAB before and after heat treatment at 300 °C for 60 minutes.

Figure 2 shows the hardness values of electroless Ni-P and Ni-P with nanodiamond coatings compared with the electroplated Ni-B and Ni-B with nanodiamond coatings. Hardness of the Ni-P and Ni-B coatings increases with the heat treatment. Heat treatment facilitates a precipitation-hardening in the Ni (B or P) coatings making them to become harder than the as deposited coatings. The increase in hardness following the heat treatment in the case of Ni-B coatings is attributed to Ni3B precipitation strengthening, while in the case of Ni-P coatings it is due to Ni3P precipitation strengthening. The hardness of as-deposited Ni-P and Ni-B coatings and after-heat treatment increases with the diamond content (Figure 2). The benefit of heat treatment on hardness and wear resistance of the composite coatings is related to an increase in the matrix-particle adhesion strength. We have proven that earlier in our publications [8] through observing the scratch marks behavior with load and speeds for coatings before and after heat treatments. We observed that the preferential crack propagation along the diamond-Ni interface is obvious in the case of the as-deposited coating. Under similar loads, crack propagation is much less pronounced in the heat-treated coatings, which contributes into increasing their hardness and wear resistance.
A concern in replacing monolithic materials with composites is the potential negative impact of the second phase on corrosion. A second phase affects the corrosion resistance because it is electrically different from the base material (the matrix) and it introduces the interphase boundaries that may favor the diffusion of anions or cations and accelerate the dissolution of the matrix. When the dispersed phase is a dielectric ceramic, as is the case of the nanodiamond used here, localized galvanic effects are not active; however, the metal-ceramic interface may still lead to a preferential path for the diffusion of aggressive ions.
The corrosion behavior of the Ni-P/Ni-B MMnC coatings prepared has been assessed by cyclic polarization in 3.5% NaCl solutions. The anodic and cathodic polarization curves are shown in Figure 3. Corrosion potential Ecorr obtained from the polarization curves in Figure 3 shows no detrimental impact on the corrosion resistance resulting from incorporating the nanodiamond.
Figure 3: Anodic and cathodic polarization curves for heat-treated electroless prepared Ni-P, Ni-P-nD and heat-treated electroplated Ni-B and Ni-B-nD samples. For the sake of clarity we have presented only the initial anodic and cathodic curves of the cyclic polarization plots, and omitted the return part of the curves.

The corrosion potential of the nanocrystalline high-P coatings increases with the increasing diamond content, particularly after heat treatment. Incorporation of nano-crystalline diamond is more disruptive, improving the corrosion resistance.
The corrosion potential of the prepared films decreases in the following order:
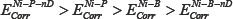
This nobility order correlates well with the texture of the deposited films rather than with the interphase boundary area.
Nanodiamond strongly influences the growth texture, the final microstructure of the coatings, and, as shown in Figure 3, affects the corrosion potential in Ni-P nanocomposite coatings. The impact of nanodiamond on the corrosion potential of the Ni-B coatings is not nearly as strong as in the case of Ni-P. In a recent publication [17], we demonstrated that the improvement of the corrosion resistance of Ni-P due to the incorporation of the nanodiamond was related to the change of the dissolution mechanism from intergranular to uniform. This effect does not seem to be operative in the case of the Ni-B coatings.
Summary
We have demonstrated that diamond particles play important role in the Ni-based protective coatings. They are especially critical for increasing the hardness of the coatings and versatile in preparing role in the Ni-based protective coatings. They are especially critical for increasing the hardness of the coatings and versatile in preparing the coatings with different dopants such as boron and phosphorus to increase the hardness. It was shown that these coatings can be prepared by different deposition processes, such as electroplating and electroless. Nanoparticles disrupt the columnar growth of Ni-P, leading to improved corrosion resistance. The impact of those particles on the corrosion resistance of Ni-B coatings is practically inexistent.
Acknowledgement
The authors would like to thank Baker Hughes, a GE Company for the support.
References
- Asrar N (2010) Corrosion control of drilling tools through chemical treatments-effectiveness and challenge. SPE Conference paper no. 130515.
- Low CTJ, Wills RGA, Walsh FC (2006) Electrodeposition of composite coatings containing nanoparticles in a metal deposit, Surf Coatings Technol 201(1-2): 371-383.
- Casati R, Vedani M (2014) Metal matrix composites reinforced by nano- particles-a review. Metals 4: 65-83.
- Ogihara H, Miyamoto K, Udagawa K, Saji T (2011) Electrodeposition of superhard Ni-B/Diamond composite coatings. Chem Lett 40(10): 10721073.
- Polushin NI, Kudinov AV, Zhuravlev VV, Stepareva NN, Maslov AL (2013) Dispersed strengthening of a diamond composite electrochemical coating with nanoparticles. Russian Journal of Non-Ferrous Metals 54(5): 412-416.
- Lee WH, Tang SC, Chung KC (1999) Effects of direct current and pulse- plating on the co-deposition of nickel and nanometer diamond powder. Surf Coatings Technol 120-121: 607-611.
- Wang L, Gao Y, Xue Q, Liu H, Xu T (2005) Effects of nano-diamond particles on the structure and tribological property of Ni-matrix nanocomposite coatings. Materials Science and Engineering A 390(1-2): 313-318.
- Monteiro OR, Murugesan S, Suresh R, Khabashesku V (2016) Corrosion- and erosion-resistant metal matrix nanocomposite coatings for the oil and gas industry. SPE Conference paper no. 179933.
- Monteiro OR, Murugesan S, Khabashesku V (2015) Electroplated Ni-B and Ni-B metal matrix diamond nanocomposite coatings. Surface Coatings Technol 272: 291-297.
- Murugesan S, Monteiro OR, Khabashesku V (2016) Extending the lifetime of oil and gas equipment with corrosion and erosion-resistant Ni-B nanodiamond metal matrix nanocomposite coatings. Offshore Technology Conference, paper no. 26934.
- Sudagar J, Lian J, Sha W (2013) Electroless nickel, alloy, composite and nano coatings-A critical review. J Alloys and Compounds 571: 183-204.
- Ogihara H, Udagawa K, Saji T (2012) Effects of boron content and crystalline structure on hardness in electrodeposited Ni-B alloy films. Surf Coatings Technol 206(11-12): 2933-2940.
- Bekish YN, Ponzyak SK, Tsybulskaya, Gaevskaya TV (2010) Electrodeposited Ni-B alloy coatings: Structure, corrosion resistance and mechanical properties. Electrochim Acta 55(7): 2223-2226.
- Balaraju JN, Sankara Narayanan TSN, Seshadri SK (2003) Electroless Ni-P composite coatings. J Applied electrochemistry 33(9): 807-816.
- Ashassi-Sorkhabi H, Es'haghi M (2013) Corrosion resistance enhancement of electroless Ni-P coating by incorporation of ultrasonically dispersed diamond nanoparticles. Corrosion Sci 77: 185193.
- Levin BF, DuPont JN, Marder AR (2000) The effect of second phase volume fraction on the erosion resistance of metal matrix composites. Wear 238: 160-167.
- Monteiro OR, Suresh R, Murugesan S, Khabashesku V (2017) Corrosion Resistance of Ni-Metal Matrix Composite Coatings: Effect of Microstructure. NACE Corrosion Conference, paper no. 9806.
© 2017 Sankaran Murugesan, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






