- Submissions

Full Text
Researches in Arthritis & Bone Study
Is DAS28 ESR Numerical Data Interchangeable with DAS28CRP?
John A Goldman*
American College of Rheumatology, USA
*Corresponding author:John A Goldman, American College of Rheumatology, USA
Submission: October 09, 2025;Published: November 07, 2025
Volume2 Issue2November 07, 2025
Opinion
I have been concerned that the ACR criteria for clinical guidance indicate that the numerical results for DAS28 ESR and DAS2CRP are interchangeable. This came to light in 2011 when a paper by Anderson JK, Zimmerman L, Kaplan L, Michaud K, wrote about the Measures of Rheumatoid Arthritis Activity in the journal Arthritis Care and Research 2011; 63: S14-36 [1]. As I read that paper more, I was appalled about such a statement. You can write a letter to the editor, but it’s limited in the amount of space you have if it is accepted (Figure 1) [2].
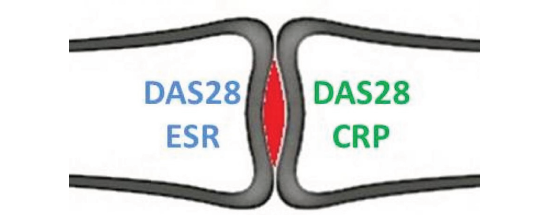
Figure 1:Ongoing inflammation in the joint being torn between DAS28ESR and DAS28CRP [2] Cush also confirms that they are not interchangeable in his article on RheumNow [2].
European Medicines Agency
“Assessment of efficacy. In general, combined measures reflecting the different signs and symptoms are to be used to document efficacy. For this purpose, diverse validated composite disease activity scores could be used, such as DAS28-ESR and DAS28-CRP, Simplified Disease Activity Index (SDAI) or Clinical Disease Activity Index (CDAI)), with established cut-off criteria for remission {DAS28-ESR or-CRP<2.6, this indicates interchangeability} SDAI≤3.3, CDAI≤2.8) and Low Disease Activity (DAS28-ESR or-CRP<3.2, SDAI≤11, CDAI≤10). DAS-28- ESR or DAS-28-CRP is commonly used to monitor disease activity of patients in practice” [3].
“However, it is acknowledged that there are some limitations in these disease activity scores and patients may still have ongoing inflammation at remission e.g. defined by DAS28- CRP. Therefore, more stringent remission criteria were developed by the ACR-EULAR. These criteria consist either of a Boolean definition, including tender and swollen joint counts≤1, and CRP≤1mg/dl, or an index-based definition, with SDAI≤3.3. In addition to the aforementioned targeted endpoints, ACR response criteria (e.g. ACR20, ACR50, ACR70 reflecting improvement of signs and symptoms from baseline of 20, 50 or 70%) should be documented” [3].
FDA Considerations for RA Drug Development
“While the FDA recognizes the Disease Activity Score in 28 joints (DAS28) as an endpoint for Rheumatoid Arthritis (RA) drug trials, there is no specific FDA guidance indicating a preference for the C-reactive protein (DAS28-CRP) version over the erythrocyte sedimentation rate (DAS28-ESR) version. However, studies have consistently shown that the two versions are not directly interchangeable and have different cut-off points for classifying disease activity.
The FDA’s general guidance for RA drug products acknowledges
composite endpoints like the DAS28 but does not mandate a specific
inflammatory marker.” “The key considerations for drug developers
include:
A. Defining endpoints: Drug developers must clearly define
their endpoints for assessing efficacy, including whether they
use DAS28-CRP or DAS28-ESR.”
B. Validation: It is the responsibility of the trial sponsor
to justify and validate the specific disease activity measure
chosen.
C. Endpoint measures: Other acceptable efficacy
endpoints in RA trials, often used in conjunction with DAS28,
include American College of Rheumatology (ACR) response
criteria, which have been shown interchangeably, as we noted,
radiographic progression, and patient-reported outcomes” [4].
When Wells G et al. [5] validated both the DAS28ESR and the DAS28CRP they felt that the DAS28CRP was better. But they were using the score of <2.6 for both, rather than an appropriate score of <2.3 for DAS28CRP [5]. DAS28-CRP underestimates disease activity when using cut-off points validated for DAS28- ESR; therefore, DAS28-ESR cut-off values should not be applied to DAS28-CRP. Although DAS28-CRP and DAS28-ESR cut-offs for LDA ≤3.2 correspond to SDAI LDA, neither corresponds well to SDAI remission.
Fleischman also Noted That
DAS28-CRP underestimates disease activity when using cutoff points validated for DAS28-ESR; therefore, DAS28-ESR cut-off values should not be applied to DAS28-CRP. Although DAS28-CRP and DAS28-ESR cut-offs for LDA≤3.2 correspond to SDAI LDA, neither corresponds well to SDAI remission [6]. The DAS28-CRP and DAS28-ESR are not interchangeable, and different cut-off points should be used for each [7].
Siemons Noted
“On average, DAS28-CRP scores were 0.20 points lower than DAS28-ESR scores, and data stratification on age and gender showed that this systematic bias was most severe in older women (0.39 points). The overall classification agreement across DAS28 categories was 76.69%, with the agreement being lowest (35.37%) in the low disease activity group. Patients were more easily classified as being in remission when using the DAS28-CRP measure. DAS28- ESR and DAS28-CRP scores are not interchangeable between individuals. The DAS28-CRP tends to yield lower values of disease activity than the DAS28-ESR, resulting in substantial classification differences [8].
Sengul noted that there is a discordance between DAS28ESR and DAS28CRP when the validated thresholds for DAS28ESR are applied to DAS28CRP. This led to errors in the determination of disease activity which can lead to errors in the management of patients with rheumatoid arthritis [9].
I even communicated with Piet Van Riel in the Netherlands, one of the originators of DAS, to see if he would agree to ask about the status of DAS28CRP compared to DAS28ESR [3]. His response is gratifying, [10].
“As you know the CRP and ESR are two different options to assess an acute phase response. The CRP will respond immediately to an acute phase response and will decrease rapidly after normalisation of the acute phase response. This is in contrast with the ESR, the increase in the ESR is more slowly after beginning an acute phase response and will also decline more slowly. In addition, the ESR is influenced by other factors like blood composition (hemoglobin and protein level) erythrocytes form. This means that in RA the ESR gives an impression of the disease activity over roughly the last week while the CRP gives information about the disease activity at the time the CRP was assessed. Sometimes this can be of help in an individual patient: for instance, ESR is elevated while CRP is normal, and there are no active joints: ESR could be explained by an infection week before which still causes an elevated ESR while the CRP is normal. So, these two measures are assessing disease activity but not completely in the same way so that’s why they might give different information in the same patient at the same time. That is also the reason why you should compare disease activity in a patient with either the DAS28CRP or the DAS28ESR, they are not completely interchangeable” [11].
When reviewing clinical studies that include all tests, such as DAS28CRP and DAS28ESR, the discrepancy becomes very clear. Be careful not to call it remission, although sometimes that is done. If you look at the data, the criteria for remission with DAS28 ESR is <2.6, while for DAS28CRP, it’s closer to <2.3. This allows authors off the hook and they can say that their papers show a DAS28CRP of <2.6 and that’s how efficient their drug is. But this is not true remission; it’s low disease activity according to DAS28CRP. They should clearly state that!
Ultimately, the decisions in the studies depend on the certifying agencies, like the European Medicines Agency and in the United States, the Federal Drug Administration, and in both include the pharmaceutical company. There are many other criteria that are being used but for those in practice, the DAS criteria may be one of the best for us. Also of caution, we have noted that the best Westergren Sedimentation Rate Is the one performed Stat in the office versus the one sent to a central lab in which there is moderate or major delay in handling and processing [12].
References
- Anderson JK, Zimmerman L, Caplan L, Michaud K (2011) Measures of rheumatoid arthritis activity. Journal Arthritis Care and Research 63(Suppl 1): S14-S36.
- Cush J (2015) DAS28-CRP or DAS28-ESR: Are they biosimilar? RheumNow.
- Committee for Medicinal Products for Human Use (CHMP) (2017) Guideline on clinical investigation of medicinal products for the treatment of rheumatoid arthritis. European Medicines Agency, Amsterdam, Netherlands.
- FDA (2013) Guidance for industry rheumatoid arthritis: Developing drug products for treatment, FDA, Maryland, USA.
- Wells G, Becker JC, Teng J, Dougados M, Schiff M, et al. (2009) Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis 68(6): 954-960.
- Fleischmann R, Desiree VDH, Koenig AS, Pedersen R, Szumski A, et al. (2015) How much does disease activity score in 28 joints ESR and CRP calculations underestimate disease activity compared with the simplified disease activity index? Ann Rheum Dis 74(6): 1132-1137.
- Moreland LW, Paulus HE, Pincus T, Russell AS, Wilski KR, et al. (2001) Consensus recommendations for the assessment and treatment of rheumatoid arthritis. J Rheumatol 28(6): 1423-1430.
- Messelink MA, Broeder DAA, Marinelli FE, Michgels E, Verschueren P, et al. (2023) What is the best target in a treat-to-target strategy in rheumatoid arthritis? Results from a systematic review and meta-regression analysis. RMD Open 9(2): e003196.
- Siemons L, Vonkeman HE, Klooster TPM, Piet LCMVR, De Mart AFJVL (2014) Interchangeability of 28-joint disease activity scores using the erythrocyte sedimentation rate or the C-reactive protein as inflammatory marker. Clin Rheumatol 33(6): 783-789.
- Sengul I, Yalbuzdag SA, Ince B, Karatepe AG, Kaya T (2015) Comparison of the DAS28-CRP and DAS28ESR in patients with rheumatoid arthritis. Int J Rheum Dis 18(6): 640-645.
- Reil VR (2025) Confidential correspondence.
- Goldman JA, Parris GR, Goldman MJ (2025) The westergren sedimentation rate: Is automation undermining clinical accuracy? ACR Convergence.
© 2025 John A Goldman. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






