- Submissions

Full Text
Polymer Science: Peer Review Journal
Wavelet-Transform Assisted Delineation of Ternary Interactions in Eu(III) - Humic Acid–Alumina Sorption System: An X-Ray Absorption Fine Structure Spectroscopy Study
Sumit Kumar1,2* and Sharayu Kasar3
1Radioanalytical Chemistry Division, Bhabha Atomic Research Centre, India
2Homi Bhabha National Institute, India
2Environmental Radionuclides Research Group, National Institutes for Quantum and Radiological Sciences and Technology (QST), Japan
*Corresponding author:Sumit Kumar, Radioanalytical Chemistry Division, Bhabha Atomic Research Centre and Homi Bhabha National Institute, Anushaktinagar, Mumbai-400 094, India
Submission: July 01, 2025;Published: August 06, 2025

ISSN: 2770-6613 Volume6 Issue 1
Abstract
Identification and characterization of the surface interactions of environmental contaminants is important for predicting their speciation and mobility. Presence of naturally occurring macro-molecular organic complexant, such as humic acid, which interacts strongly with the metallic contaminants as well as various mineral surfaces, complicates the delineation of these interactions and the responsible surface species. X-Ray Absorption Fine Structure Spectroscopy (XAFS), being an element specific probe, has been extensively used for the local structure characterization of the surface species. However, strong dependence of backscattering amplitude on the atomic number of the backscatters causes problem in identifying overlapping interactions. Herein, XAFS analysis was assisted by wavelet transform of XAFS data to identify the ternary interactions in the alumina-humic acid-Eu(III) sorption system. Under the varying addition order of HA and Eu(III) to the alumina suspension at pH ~6, both the two ternary sorption systems indicated the existence of two ternary species, one having HA and the other Eu as the bridging component. The ternary sorption system in which HA was first equilibrated with alumina before Eu, showed higher formation of HA bridged species and vice versa. Application of wavelet transform to identify interactions and using this information for XAFS analysis is thus a potent technique for environmental studies.
Keywords:Europium; Sorption; Alumina; Humic acid; XAFS Wavelet-transform
Opinion
Humic Acid (HA), a high molecular weight naturally occurring polydisperse organic complexant, exhibits high affinity for metal ions owing to its multiple functional groups (Figure 1), [1,2]. They thus govern the speciation and mobility of naturally occurring and anthropogenic metal ions. Another controlling aspect in mobility of environmental contaminants is their interaction with mineral surfaces. This mineral interaction, however, becomes complicated in the presence of HA; HA has been found to strongly interact with the mineral surface through ionic and ligand exchange process [2]. The affinity of metal ions with surface bound HA has been found stronger compared to that present in the aqueous phase [3]. Thermodynamic description of the metal ion retention by mineral surfaces in presence of HA thus often deviate from the calculation based solely on the additivity of the Metal ion - HA and Mineral surface - HA interaction models [4]. Macroscopic experiments under varying chemical conditions along with spectroscopic techniques, such as luminescence spectroscopy have been carried out to delineate the chemical interactions of the sorbing metal ions on oxides and clays surface in presence of humic acid [5]. These studies provide indications towards the existence of ternary metal ion-HA-mineral surface interactions; however, no direct evidence regarding ternary species responsible for the sorption behaviour of metal ions has been reported..
Figure 1:Stress-strain curves [43]: (a) Tensile behavior at different strain rates and room temperature, (b) Tensile behavior at three temperatures and 0.001s-1.
X-ray Absorption Fine Structure Spectroscopy (XAFS) is an element specific probe that excites an inner core electron on excitation with X-ray of suitable energy [6]. The quantum interference pattern of such excited photoelectron on scattering with the surrounding atom over an X-ray energy range of ~ 1 keV from the excitation energy of zero kinetic energy photoelectron (i.e., the absorption edge) constitutes the XAFS spectra representing the local coordination environment around the probe atom. As the basic data crucially depends on the backscattering of the photoelectron with the surrounding atom, the similar scattering ability of nearby-atomic-number atoms and of low atomic number limits the resolution on identification of the surrounding atoms. To get rid of this problem, Wavelet Transform (WT) of the XAFS data is proposed wherein magnitude of XAFS data is plotted as a function of two parameters (k, R). This helps in resolving the multitude of the interactions happening in the local environment based on atomic number of the scattering atoms [7]. It is note here that WT, being a qualitative tool for the analysis of XAFS data, aids in the identification of spectral features, even in the presence of noise or complex contributions like multiple-scattering pathways or multielectronic excitations.
In this study, Eu(III) sorption on γ-alumina was studied using batch sorption and Eu-L3 edge XAFS spectroscopy under varying addition order of metal ion and HA to the alumina suspension in groundwater pH condition. Aim is to identify the surface interactions of Eu(III) and calculate the structural details of the surface species involved in those interactions. Wavelet transform and conventional XAFS analysis were carried out for the work. Eu(III) was chosen as the analogue of trivalent lanthanides and actinides.
Experimental
Materials and characterization
γ-Alumina powder from Degussa India Ltd. was used as obtained. The particle size determined by N2 gas-based BET analysis, was found to be 203m2/g. HA was sourced from M/s. ACROS Co., as sodium salt and was characterized for total Proton Exchange Capacity (PEC) following standard Baryta method [2]. PEC of HA was found to be 5.67meq/g. All the other chemicals used in the experiment were of analytical grade and procured from the local suppliers.
Batch sorption studies
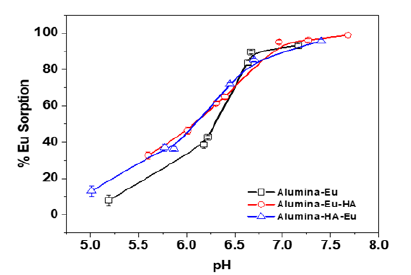
Figure 2:Eu(III) sorption on alumina in absence and presence of humic acid. [Eu(III)]=5×10-5M, [HA]=2mg/L, suspension strength=1g/L, sorption system background electrolyte 0.1M NaCl.
Alumina suspensions (1g/l) were prepared in polypropylene
tubes and equilibrated overnight for saturation of the surface
reactions of the alumina particles with aqueous medium (0.1M
NaCl). In macroscopic sorption systems, 5×10-5M Eu(III) ions
were contacted with alumina suspension with and without HA
(2mg/l) as a function of pH (5-8). Addition order of HA and Eu(III)
was varied making two ternary systems, e.g. Alumina-Eu-HA (cf.
Figure 2) label indicates that Eu(III) was first equilibrated with the
Alumina suspension before the equilibration of the binary system
with HA. Equilibration with Eu(III) ions and HA were done for
24 and 48 hours, respectively. Eu estimation before and after the
final equilibration was carried out using Eu-154 radioisotope and
NaI(Tl) detector (well type 3”×3” scintillation detector coupled
to 1024 channel analyzer). Herein, Eu(III) stock solution used
in the study was doped with Eu-154 radiotracer solution. After
equilibration, pH of the suspensions was measured and suspensions
were centrifuged at 16000rpm for 45min. Two 1ml aliquots from
each tube were taken for gamma activity measurement of 154Eu;
the measured activity represents the Eu(III) left in the supernatant
after equilibration. The percentage sorption was calculated
from the measured activity data before and after the solid phase
separation as,
% sorption=[(A0-A)/A0]×100 (1)
where, A0 and A represent 154Eu activity before and after the
phase separation of the suspensions.
XAFS sample making and data acquisition
The solids separated from the ternary sorption systems were air dried and mixed with polyvinyl pyrrolidone to make pallet and was used for the XAFS measurement. The Eu L3 XAFS measurements were performed in the fluorescence mode at the XAFS beam line at Elettra synchrotron facility. The spectra were collected over the energy range 6700-7600eV, focusing on the Eu L3 edge energy (6977eV), with a pre-edge energy step of 5eV, 0.20eV in the edge region and thereafter a constant k step of 0.03 Å-1 in the extended XAFS region. Typically, three scans were taken for each sample and the data could be obtained in the photoelectron momentum space extending up to 10 Å-1. Though the energy range provides data upto ~13 Å-1, higher noise at high k values restricted the analysis upto 10 Å-1. Higher energy range allows higher resolution in R values; the choice of lower values, in the present analysis, though would not affect adversely as the distant Eu-Al shells are quite apart. The conventional analysis of the Eu(L3) edge XAFS spectra for the samples in the extended energy range was carried out by the Demeter suit of softwares [8].
Wavelet transform (WT) analysis
A Morlet wavelet transform analysis (HAMA software) was used to identify various shells prior to analysis of the Extended- XAFS (EXAFS) spectra of the sorption samples as discussed in the previous section [7]. This qualitative analysis presents the filtered wavelet-transformed XAFS amplitude as a function of k and R (uncorrected for phase shift). Resolution of amplitude in two dimensions facilitates the identification of individual contributions in k-space to each peak in the Fourier transform modulus and is therefore better suited to distinguish between heavier and lighter backscattering atoms, even if they are at the same distance from the central atom. As the aim in this analysis was only to identify different scatters producing different chemical interactions, overview WT of the whole spectral range was generated using Morlet wavelet parameters η=15 and σ=1. In this asymptotic case the wavelet ordinate R and the Fourier abscissa R become equal.
Results and Discussion
Batch sorption behaviour
Figure 2 presents Eu(III) sorption percentage at pH ~6.0 in presence and absence of HA and under varying addition order of HA and Eu(III) to the alumina suspension. Herein compared to the binary system, irrespective of the addition order percentage sorption of Eu(III) in presence of HA is enhanced at lower pH. Beyond pH ~7, there was no difference in Eu(III) sorption in the binary and ternary sorption systems. The higher sorption in the lower pH range could be attributed to the presence of HA on the alumina surface, which is in concurrence with the higher sorption of anionic species and ligand in the acidic to neutral pH range [9].
Wavelet transform analysis of the binary and ternary sorption systems
Figure 3:Wavelets transform of the XAFS spectra of Eu-HA-alumina system. SiEu and (SiEuHA and SiHAEu) are the binary and ternary sorption systems. Eu-HA represents the Eu(III) equilibrated with HA under identical pH and concentration condition like the sorption system.
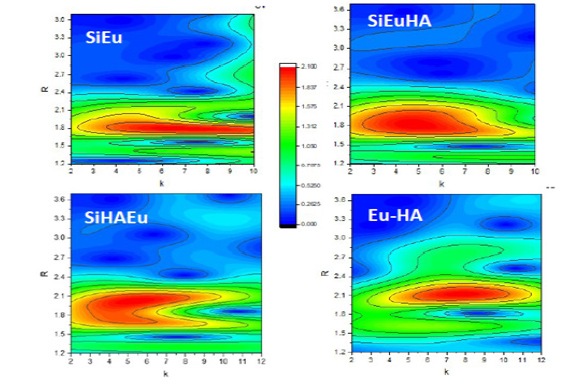
Experimental conditions of the sorption samples analysed using XAFS spectroscopy is given in Table 1. Figure 3 presents the wavelet transform analysis of the sorption systems. For comparison, Eu(III) contacted with HA solution was also analysed and presented in the Figure 3. Figure 3 shows the WT representations we obtained for the binary and ternary sorption systems. For the binary system of Eu(III) sorbed on alumina (SiEu) there was a lobe corresponding to R value ~1.8 Å spread over Δk=4-10 Å-1 and it was centred around ~ 7 Å-1. For Eu(III)-HA interaction system (Eu-HA), bonding corresponded to R ~ 2.1 Å and the Δk window (5-10 Å-1) was centred around ~ 7.5 Å-1. Interestingly, in both the ternary systems, signatures of both the binary systems were found present though the features in k space were found shifted to lower values (Figure 3). In SiEuHA, Eu(III) mainly interacted with alumina surface like (SiEu) system and it might be possible that surface sorbed HA interacted with additional dissolved Eu(III) to create the signal like the Eu-HA system. In the ternary system wherein HA was added first, main interaction would be similar to Eu-HA system and this was found stronger in the SiHAEu WT signal. This qualitative analysis therefore clearly established that irrespective of the addition order, both HA mediated binding (alumina-HA-Eu) and metal ion mediated binding (alumina-Eu-HA) exist in the ternary systems.
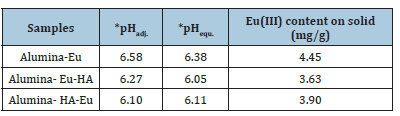
Table 1:Experimental condition of the XAFS samples. In the sample name, order of components (left to right) represents the sequential equilibration order in the suspension. *pHadj. and pHequ. are the pH values adjusted and observed on equilibration of the oxide suspension with Eu(III) and HA.
Extended XAFS analysis
Figure 4:EXAFS data and their fourier transform in R-space; (a, c) for the Alumina-Eu-HA system and (b,d) for the alumina-HA-Eu system.
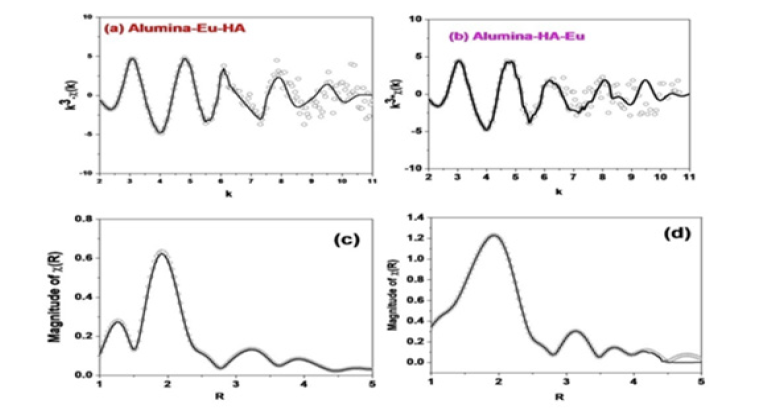
Extended XAFS (EXAFS) data for the ternary systems were Fourier transformed to get the data in the R-space and as per the conventional methodology of analysis [6], the data was modelled in the R space to delineate the bonding interactions. Figure 4 presents the EXAFS data and their Fourier transformed R-space data for the ternary systems. Based on the similarity of wavelet feature and the HA being only complexant in the present study, it is indicative to consider Eu(III) - Carbon atom as one of the backscattering shell in XAFS quantitative analysis; in more complex systems, this type of choice may not be so direct and the support from the other spectroscopic technique would be needed in making the choice of backscattering shells. Table 2 gives the metric result of the analysis of EXAFS spectrum. To compare ternary system interactions with that of the binary system (SiEu), results from the earlier study [10] are also added in the Table 2. Herein, scattering path represents the path of the photoelectron which originates from the probe atom (Eu) and returns to it after scattering from surrounding atoms, such as, O, C, Al. Corresponding to a particular path, R represents the radial distance between two atoms and the no. of such backscattering atoms is given by N (the amplitude factor S02 is kept fixed at 1). Disorder corresponding to a particular scattering path is given by debye-waller factor (σ2) and the energy scale difference between theory and model is given by ΔE. The quality of fit factor is Rf.
Table 2:EXAFS metric result for the neighbouring shells contributing to the EXAFS spectra of the binary and ternary sorption systems in Alumina-Eu-HA system. Number in the parenthesis represents the error on the last digit of the metric value.
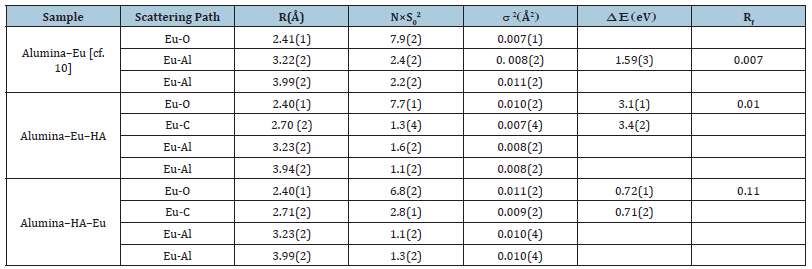
The R value for the Eu-O coordination shell indicates the similarity of primary binding in binary and ternary sorption systems. However, N value is smaller for the ternary systems and among the ternary systems, smaller in that system which has HA as mediator between alumina and Eu (Alumina-HA-Eu). The higher Debye Waller factor (σ2) for the alumina-Eu(III)-HA system is attributed to higher heterogeneity owing to the complex structure of HA. This higher value contributes to the lowering of the coordination of oxygen atoms around Eu(III) in ternary system. Like the binary systems, in both the ternary systems Eu-Al1 and Eu-Al2 coordination shells (R value ~ 3.22 and 3.99 Å) were found in the local structure of the Eu(III) atom. Apart these shells, Eu-C coordination shells at R ~ 2.70 Å was observed; the contribution of this shell was found higher, in concurrence with the wavelet transform analysis, in the ternary system wherein HA was first equilibrated with the alumina surface.
Conclusion
The existence of ternary interactions, wherein one component bridges the other two components of the sorption system, has been indicated in various macroscopic and spectroscopic studies involving IR and fluorescence investigation. As these interactions cause the sorption characteristics to be beyond the additivity of the binary systems, modelling of the contaminants sorption characteristics and thereby their mobility in environment requires characterization of the ternary species. The present study used wavelet transform assisted analysis of the x-ray absorption fine structure spectroscopy data for the alumina-humic acid-Eu(III) ternary sorption system to identify and characterize the species. This approach is very potent wherein overlapping species existence, such as competitive complexation/sorption sites, is expected and will be very useful for speciation studies in the environmental systems.
Acknowledgement
Author acknowledges the support and encouragement of Head, RACD and Head, CCS, RACD, BARC during the work.
References
- Stevenson FJ (1982) Humus chemistry, genesis, composition, reactions. Journal of Chemical Education 72(4): A93.
- Bryan ND, Abrahamsen L, Evans N, Warwick P, Buckau G, et al. (2012) The effects of humic substances on the transport of radionuclides: Recent improvements in the prediction of behaviour and the understanding of mechanisms. Appl Geochem 27(2): 378-389.
- Reiller P, Casanova F, Moulin V (2005) Influence of addition order and contact time on thorium (IV) retention by hematite in the presence of humic acids. Environ Sci Technol 39(6): 1641-1648.
- Kumar S, Rawat N, Kar AS, Tomar BS, Manchanda VK (2011) Effect of humic acid on sorption of technetium by alumina. J Hazard Mater 192(3): 1040-1045.
- Yan M, Korshin GV (2014) Comparative examination of effects of binding of different metals on chromophores of dissolved organic matter. Environ Sci Technol 48(6): 3177-3185.
- Konigsberger DC, Prince R (1988) X-Ray absorption: principles, applications, techniques of EXAFS, SEXAFS and XANES. Wiley, New York, USA.
- Funke H, Scheinost AC, Chukalina M (2005) Wavelet analysis of extended X-ray absorption fine structure data. Phys Rev B-Condens Mater Phys 71: 094110.
- Ravel B, Newville M (2005) ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J Synchrotron Radiat 12(4): 537-541.
- Fairhust AJ, Warwick P, Richardson S (1995) The influence of humic acid on the adsorption of europium onto inorganic colloids as a function of pH. Coll Surf A 99(2-3): 187-199.
- Kumar S, Kar AS, Tomar BS, Bhattacharya D (2012) X-ray absorption fine structure spectroscopy study of Eu (III) sorption products onto amorphous silica and γ-alumina: Effect of pH and substrate. Polyhedron 33(1): 33-40.
© 2025 Sumit Kumar. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






