- Submissions

Full Text
Polymer Science: Peer Review Journal
Beta-Cyclodextrin: A Cyclodextrin Derivative and its Various Applications
Noor Fatima1, Syed Haroon Khalid1, Kiran Liaqat1, Ali Zulfiqar2 and Rabia Munir1*
1Department of Pharmaceutics, Faculty of Pharmaceutical Sciences, Government College University, Faisalabad, 38000, Pakistan
2National University of Science and Technology, Islamabad, Pakistan
*Corresponding author:Rabia Munir, Department of Pharmaceutics, Faculty of Pharmaceutical Sciences, Government College University, Faisalabad, 38000, Pakistan
Submission: February 15, 2023;Published: March 27, 2023

ISSN: 2770-6613 Volume4 Issue5
Abstract
Cyclodextrin are useful functional excipients that have enjoyed widespread attention and use. The basis for this popularity from a pharmaceutical standpoint is the ability of these materials to interact with poorly water-soluble drugs and drug candidates water solubility. β-cyclodextrins have a wide range of applications in different areas of drug delivery and pharmaceutical industry due to their complexation ability and other versatile characteristics. β-cyclodextrin (βCD) contains 7 glucopyranose units and is the most commonly used cyclodextrin polymer. They are known to form inclusion complexes with poorly soluble drugs and to improve their bioavailability and enhance their solubility. The objective of this review is to summarize general properties and different uses of βCD, and recent advancements related to its application.
Keywords:Cyclodextrin; Drugs; Glucose; βCD molecule; Antioxidants
Introduction
Cyclodextrins are hollow, truncated cone-shaped cyclic oligosaccharides. The distinct chemical structure of cyclodextrin enables them to entrap poorly water-soluble drugs including antioxidants, into their cavities, forming inclusion complex resulting in a significant improvement in the stability and solubility of drugs. CDs are produced as a result of the degradation of starch, corn, potato by enzymes, in which residues of glucose link with each other by -1,4 glycosidic linkages and then result in formation of a macro-cycle. It yields a combination of linear, branched and cyclic dextrins [1].
Types of Cyclodextrins
Cyclodextrins are sugar molecules that have been linked together in rings of varying diameters. The sugar units are known as glucopyranosides-glucose molecules with a pyranose (six-membered) ring structure. Six, eight, or ten glucopyranosides combine to make α, β and γ cyclodextrin, respectively. The three cyclodextrins exist naturally; A. Villiers named them “cellulosine” when he first reported them in 1891. A. Schardinger defined the three varieties a few years later. Despite their complexity, cyclodextrins are quite simple to produce. A combination of common enzymes, most notably cyclodextrin glycosyl transferase and -amylase, is used to treat starch. Each enzyme combination generates a distinct ratio of the three cyclodextrins.
On basis of number of α glucose units, CDs are classified into three types:
a. α Cyclodextrins
b. β Cyclodextrins
c. γ Cyclodextrins
α-Cyclodextrins contain 6, β-Cyclodextrins contain 7 while γ-Cyclodextrins contain 8 units of glucopyranose linked by 1-4 bonds [2].
β-cyclodextrin ( βCD)
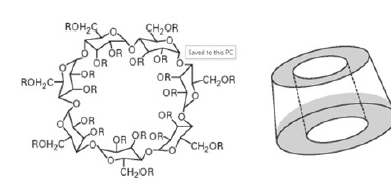
βCD is a cone-shaped molecule. Many hydroxyl groups are present on the outer surface of the cavity and its cavity is hydrophobic from inside. As a result, βCD is soluble in water and may encapsulate a wide range of hydrophobic guest molecules in its non-polar cavity. This property has been widely used in drugcontrolled release, separation and adsorption. The molecular structure of βCD is shown in Figure 1.
Figure 1:Molecular structure of βCD.
General Characteristics of βCD
βCD is the most easily available , economical and useful of all cyclodextrins. It contains 7 glucopyranose subunits linked by 1-4 glycosidic linkage. The Molecular weight of β-cyclodextrin is 1135g/mol. It has a melting point of 280 ºC. It has a cavity diameter of 6- 6.5 Aº. Cavity volume is 262 Aº 3. Its outer diameter is 15.4 Aº and the height of its torus is 7.9 Aº .Its Solubility in the water is 1.85g/100ml at 25 ᵒC. Its Pka value by potentiometry is 12.202 at 25 ᵒC. Its cavity size is ideal for most pharmaceuticals with molecular weights ranging from 200 to 800 Daltons. It forms Mono-clinic parellograms crystals from the water. A small amount, approximately 1-2 % of it is absorbed in upper GIT, no metabolism occurs in upper GIT. It is metabolized in the lower GIT by the bacterial action in the colon and caecum region [3,4].
Host Guest Interaction of βCD
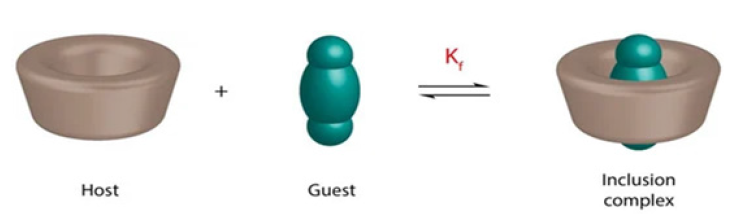
βCD is the most widely studied and commonly used cyclodextrin on the basis of affordability, accessibility, and its capability of forming complexes with an extensive range of chemicals. Like other CDs the most distinctive characteristic of βCD molecule is the capability to produce inclusion complex with numerous chemicals through a host guest interaction (Figure 2), [5]. The method of Inclusion complex formation is a widely used method for improving the solubility of substances. Its exterior is hydrophilic due to presence of 21-OH groups, inside cavity is composed of seven glucose units and is hydrophobic. The core structure has the capability to encapsulate other substances. These exceptional encapsulation qualities can alter and/or enhance the guest molecule’s biological, chemical, and/or physical capabilities [6].
Figure 2:Illustration of formation of inclusion complex of a drug (guest) and cyclo dextrin (host) [5].
Applications of βCD
CDs are used widely in pharmaceutical sciences. CDs are able
to increase stability, solubility and also bioavailability of many
bioactive hydrophobic compounds by complex formation [2]. There
are following properties of βCD,
a. βCD enhances the water solubility of various poorly
water-soluble compounds, resulting in increased bioavailability
and pharmacological impact, allowing the therapeutic dose of
drug to be reduced. It can be used to increase the stability of
compounds so that they can withstand degradation, oxidation,
temperature, light, and metal salts [7].
b. Carbohydrate-based surfactants significantly improve
their effectiveness and potential application range when
complexes with βCD and other cyclodextrin. The creation of
CD/surfactant host-guest compounds increases the critical
micelle concentration and surfactant solubility [8].
c. βCD have capability of inclusion complex formation with
antioxidants and also with the UV filters for enhancing aqueous
solubility of chemical Ultraviolet filters and antioxidants and
also for providing a shield against other degradative variables.
They can also provide a controlled drug release profile
from sunscreens. It has the potential to enhance sunscreen
effectiveness, promote stability and thus provide better
protection from UV rays [9].
d. Kfoury et al. [10] focused on improving the water solubility
and antioxidant activity of caffeic acid by complexation in
cyclodextrin or by the use of ethanol as a co-solvent. Phase
solubility studies showed solubility of the substance enhanced
in a linear mode by use of cyclodextrin and in an exponential
mode by the use of ethanol. The combined effect of ethanol
and the use of β cyclodextrin-caffeic acid inclusion complex
improved the solubilization as well as antioxidant activity
of the substance. Antioxidant activity was enhanced in the
combination system as a result of its increased solubility.
e. βCD is currently the most common cyclodextrin which
is used in pharmaceutical formulations. It can be used for
masking unpleasant odors and tastes. It can also be utilized
to camouflage the color or pigments of the substances. It
can provide protection against microbial degradation to the
substances. It can modify the chemical reactivity of its guest
molecules and is also used for the successful conversion of
liquid substances into powder form [11].
f. In nanotechnology, they can be used for formation of
nano-sponges, nanoparticles, nano-micelles, and also nanovesicles
etc. which have extensive application in nanomedicine.
For example, nano-sponges based on β cyclodextrin can be used
in delivery of anticancer agents [12].
g. In chemistry ,it can be used as chemical sensors [13]. It
can also be used in tissue engineering in preparation of tissue
scaffolds [14].
h. βCD were used for the efficient reduction of organic
pollutant such as the nitroaromatics, by use of chemistry
using catalytic materials like β-cyclodextrin functionalized
gold nanoparticles The ability of nanocatalysts made by use of
β-cyclodextrinto decrease nitroaromatics may offer promise
for detoxifying aquatic environments [15].
i. βCD has wide use in the textile industry. As they are
environmentally friendly, cost-effective, and easy to produce on
a large scale and also have physicochemical (such as inclusion
complex-forming ability, chelating function, emulsifying activity,
slow release of fragrances) and biological (e.g., biocompatibility,
biodegradability, drug - delivery ability, pesticidal delivery)
characteristics which can be used in many ways in textile
industry, including antibacterial (odour absorbing, active
drug stabilizing), fragrance, UV resistance, water resistance,
antimicrobial resistance, heat resistance, and insect repellent
[16]. It is a potential agent in textile finishing. Using cross-linking
and binding agents, cyclodextrins are grafted into materials.
Pellicer et al. [17] synthesized polymers of β- cyclodextrin
and Hydroxypropyl-β-cyclodextrin using epichlorohydrin as
crosslinking agent, which were then utilised to remove the azo
dye Direct Red 83:1. The adsorption capability of the polymer
produced from β-cyclodextrin was almost six times higher than
that of Hydroxypropyl-β-cyclodextrin indicating that β-CD-EPI
insoluble polymer is of great use for removal of dye direct red
83:1 as a viable substitute for more expensive adsorbents, easy
to prepare and less costly.
Limitation of Using βCD
There is rapid removal of drug from the bloodstream after in vivo administration. There is also a possibility that, in the presence of biological media, the entrapped drug moieties can be replaced by other molecules with a higher affinity for the CD cavity. The hollow cavity of βCD can limit the effectiveness βCD of drug carriers [18]. Some other limitations are administration routes of some CDs are limited and supergeneric strategy was not a success.
Conclusion
This article provides an overview of βCD, its general features and its uses in many domains. βCD are not only well-known solubilizers, but they are also highly effective permeability enhancers [19]. There are a lot of fascinating prospects for future uses of βCD, such as enhancing solubility, bioavailability, and stability, which may tackle many drug delivery problems via complexation. βCD has mostly been utilized in the pharmaceutical sector as a complexion agent to enhance the water solubility of poorly soluble medicines, as well as their bioavailability and stability. When a drug molecule forms a compound with βCD, a given lipophilic moiety of the drug molecule enters the hydrophobic cyclodextrin cavity, according to classical cyclodextrin chemistry.
References
- Bai Y, Liu CP, Chen D, Liu CF, Zhuo LH, et al. (2020) β-Cyclodextrin-modified hyaluronic acid-based supramolecular self-assemblies for pH-and esterase-dual-responsive drug delivery. Carbohydrate Polymers 246: 116654.
- Duchê D (2011) Cyclodextrins and their inclusion complexes. Cyclodextrins in pharmaceutics, cosmetics, and biomedicine.
- Del Valle EMM (2004) Cyclodextrins and their uses: a review. Process Biochemistry 39(9): 1033-1046.
- Muñoz-Botella S, Del Castillo B, Martin MA (1995) Cyclodetrin properties and applications of inclusion complex formation. ARS Pharm 36(2): 187-198.
- Kfoury M, Landy D, Fourmentin S (2018) Characterization of cyclodextrin/volatile inclusion complexes: A review. Molecules 23(5): 1204.
- Crini G, Fourmentin S, Fenyvesi É, Torri G, Fourmentin M, et al. (2018) Cyclodextrins, from molecules to applications. Environmental Chemistry Letters 16: 1361-1375.
- Eastburn SD, Tao BY (1994) Applications of modified cyclodextrins. Biotechnology Advances 12(2): 325-339.
- Valente, Artur JM, Söderman O (2014) The formation of host-guest complexes between surfactants and cyclodextrins. Advances in Colloid and Interface Science 205: 156-176.
- Dahabra L, Broadberry G, Le Gresley A, Najlah M, Khoder M (2021) Sunscreens containing cyclodextrin inclusion complexes for enhanced efficiency: A strategy for skin cancer prevention. Molecules 26(6): 1698.
- Kfoury M, Geagea C, Ruellan S, Greige-Gerges H, Fourmentin S (2019) Effect of cyclodextrin and cosolvent on the solubility and antioxidant activity of caffeic acid. Food Chemistry 278: 163-169.
- Singh M, Sharma R, Banerjee UC (2002) Biotechnological applications of cyclodextrins. Biotechnology Advances 20(5-6): 341-359.
- Trotta F, Dianzani C, Caldera F, Mognetti B, Cavalli R (2014) The application of nanosponges to cancer drug delivery. Expert Opinion on Drug Delivery 11(6): 931-941.
- Varca GHC, Andreo-Filho N, Lopes PS, Ferraz HG (2010) Cyclodextrins: an overview of the complexation of pharmaceutical proteins. Current Protein and Peptide Science 11(4): 255-263.
- Lukášek J, Hauzerová Š, Havlíčková K, Strnadová K, Mašek K, et al. (2019) Cyclodextrin-polypyrrole coatings of scaffolds for tissue engineering. Polymers (Basel) 11(3): 459.
- Rajamanikandan R, Ilanchelian M, Ju H (2023) β-cyclodextrin functionalized gold nanoparticles as an effective nanocatalyst for reducing toxic nitroaromatics. Optical Materials 135: 113294.
- Romi R, Nostro PL, Bocci E, Ridi F, Baglioni P (2005) Bioengineering of a cellulosic fabric for insecticide delivery via grafted cyclodextrin. Biotechnology Progress 21(6): 1724-1730.
- Pellicer JA, Rodríguez-López MI, Fortea MI, Lucas-Abellán C, Mercader-Ros MT, et al. (2019). Adsorption properties of β-and hydroxypropyl-β-cyclodextrins cross-linked with epichlorohydrin in aqueous solution. A sustainable recycling strategy in textile dyeing process. Polymers 11(2): 252.
- Mura P (2020) Advantages of the combined use of cyclodextrins and nanocarriers in drug delivery: A review. International journal of pharmaceutics 579: 119181.
- Cramer F (1954) Einschlussverbindungen: Springer, Germany.
© 2023 Rabia Munir. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






