- Submissions

Full Text
Polymer Science: Peer Review Journal
Recovery of Cellulose from Polyester/Cotton Fabrics Making Use of Ionic Liquids
Villalta Boza E, Riba-Moliner M and Lis Arias MJ*
INTEXTER-UPC Colon, 15 08222 Terrassa, Barcelona, Spain
*Corresponding author:Lis Arias MJ, INTEXTER-UPC Colon, 15 08222 Terrassa, Barcelona, Spain, ORCID Number: 0000- 0002-2026-085X M Riba Moliner ORCID Number: 0000-0003-0001-2055
Submission: November 04, 2022;Published: November 29, 2022

ISSN: 2770-6613 Volume4 Issue3
Abstract
This article refers to the chemical recovery of cellulose from fabrics composed of Cotton (CO)/Polyester (PES) achieved using Ionic Liquids (ILs). Initially, the effect of ionic liquids on the surface of the textile is analyzed, determining the influencing factors related to the entry of IL inside the textile and the chemical mechanism that controls the system. This work considers the influence of the time, ratio, and temperature variables on the system, with the aim of defining which of them has a greater influence on the process. The ability of ionic liquids, specifically 1-Allyl-3-Methylimidazolium Chloride (AmimCl), to dissolve cellulose and subsequently regenerate the material through a simulation of the wet spinning process is evaluated. The responsible for the fiber’s inflation, water or DMSO, has also been another factor of study, analyzing the influence of each solvent and the interactions when in contact with the ionic liquid. Finally, the regenerated substance is characterized by its surface structure using the Scanning Electron Microscope (SEM), its molecular structure by Infrared Spectroscopy Analysis (FTIR), and its thermal stability by Thermogravimetric Analysis (TGA).
Keywords:Polyester; Cotton; Ionic liquids; Chemical recycling; Cellulose
Abbreviations:AmimCl: 1-Allyl-3-Methylimidazolium Chloride; SEM: Scanning Electron Microscope; TGA: Thermogravimetric Analysis; NMMO: N-Methylmorpholine N-Oxide
Introduction
Currently, one of the main challenges the textile sector is facing is to create an ethical, sustainable and eco-efficient industry. Different factors such as the high consumption of water, greenhouse gas emissions, the generation of microplastics and pollution from waste, have made this industry be considered one of the most polluting on the planet. Textile consumption per inhabitant is growing exponentially, a trend that natural resources and raw materials follow as a consequence. The increasing exhaustion of non-renewable raw materials is one of the main concerns, therefore the development and implementation of new processes to reduce the use of virgin resources is a key opportunity to increasing the circularity of products. If the planet has physical limits, it is fundamental to change and not exceed the capacity of our ecosystem. Thousands of tons of textile waste are thrown away in landfills or are burned without having the possibility of recycling them. All the raw materials, the volume of water used, the transport and the treatment that have been carried out on the textile products and afterwards end up being rejected, have serious consequences on the planet. This fact shows the urgent need for a change in the management of the generated waste, in order for us to adapt and be in harmony with the environment. Although responsible consumption is promoted, it is necessary to deal with all the waste generated and that will continue to be produced. Therefore, the research and development of new technologies on the management and treatment of textile waste is a key opportunity to achieve comprehensive circular economy models that ensure the closure of cycles.
It is estimated that [1] Europe generates 3 million tons of textile waste annually, of which 85% ends up in landfills or is incinerated, and the rest is recovered for reuse in the textile industry. Nowadays, the end of life of textiles is mainly focused on landfills and incinerators and, to a lesser extent, on collection points to be reused or recycled. Africa and some Asian countries have already begun to reject part of the European used clothing imports, since it does not promote the development of the local textile industry. Regarding the incineration of textiles, France has forbidden it and Spain will do it as well [2]. For this reason, there is a great opportunity in the development of recycling methods to manage more than a million tons of textile waste.
Textile recycling
In a recycling process it is necessary to identify the fibers by which the textile waste is composed. Generally, fiber blending is the predominant option in the market, either to get economic benefits or to produce fabrics with specific requirements and properties that can only be obtained through a blend of several fibers [3]. Currently, it is estimated that approximately [1] 80% of the textile waste generated is composed of cotton and polyester fibers. On the other hand, the remaining 20% refers to acrylic fibers, viscose, wool, nylon and other types of fibers textiles. The main problem of recycling a CO/PES blend is that they are composed of very different fibers, and, therefore, their behavior under the applied treatment conditions is not the same. If a depolymerization of PES is carried out, it involves the degradation of cellulose into smaller polymer fractions [3]. Chemical recycling [3] is about the decomposition of the fiber and the polymer, having the option of re-polymerizing the monomers into a new polymer. Chemical recycling is a research challenge that has to be studied to find out if it is a sustainable and viable solution that could be implemented on an industrial scale. Main circular models are those that constitute natural systems since they work in closed material cycles. Therefore, they do not generate waste but are used as raw material. For this reason, if a completely circular process wants to be achieved, it is important to imitate nature, so the proposal of a chemical treatment could have great potential.
Cellulose dissolution with ionic liquids
Cellulose is insoluble in water and in some common organic solvents due to the presence of intramolecular and intermolecular hydrogen bonds [4]. To achieve the dissolution of cellulose it is necessary to break the interaction produced by the intermolecular hydrogen bonds [5]. The fact that cellulose is insoluble in most conventional solvents opens news research possibilities into new efficient and environmentally responsible solvent systems. N-Methylmorpholine N-Oxide (NMMO) [6] is the only solvent used to manufacture cellulose fibers (Lyocell technology). This has some disadvantages and limitations [7] such as the high energy consumption to dissolve the cellulose, its low thermal stability and its high cost. Furthermore, it leads to secondary reactions, and it can cause the degradation of the cellulose. The most influential parameters [5] in the cellulose dissolution process are temperature, time, degree of polymerization (cellulose solubility can decrease with increasing degree of polymerization) and crystallinity. The crystalline regions are more energetically stable; therefore, the dissolution of the crystalline zones is more complex [8].
Ionic liquids present functional duality, meaning that they have
both the capacity of inflating the fiber and inducing the hydrolytic
rupture of the glycosidic bonds of cellulose [9], leading to the
formation of reducing sugars (Figure 1). As shown in Figure 2, the
cellulose dissolution mechanism occurs when the anion and cation
of the ionic liquid form hydrogen bonds with the hydrogen and
oxygen atoms of the cellulose [10]:
A. Hydrogen bond between the oxygen of the OH group of the
cellulose and the cation of the IL [11]. Cellulose oxygen atoms
act as electron pair donors while cations of the IL act as
electron acceptors [12].
B. Hydrogen bond between the hydrogen of the OH group of the
cellulose and the anion of the IL [11]. Hydrogen atoms act as
electron acceptors and the anion as an electron donor [12].
Figure 1: Cellulose o-glycosidic bond [10].
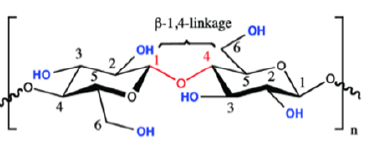
Figure 2: Cellulose dissolution mechanism in ionic liquid [13].
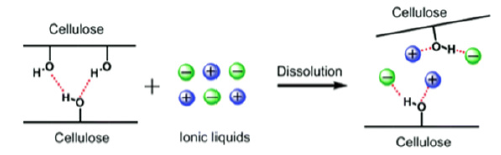
It is very important that atom centers are located close
enough to allow interactions and the formation of donor-acceptor
complexes [12]. After the interaction of cellulose and the IL, the
oxygen and hydrogen atoms of the hydroxyl groups separate, and
as a result, open the hydrogen bonds between the molecular chains,
achieving the dissolution of the cellulose [13]. In the article “The
influence of the cation type of ionic liquid on the production of
nanocrystalline cellulose and mechanical properties of chitosanbased
biocomposites” it has been proven that the cation can play a
significant role in the dissolution process. This mechanism involves
the synergistic interaction of the cation and anion of the IL [14]:
a) First of all, the anions bind to the hydroxyl groups on
the cellulose surface, causing a weakening of the hydrogen bonds
between the cellulose chains. Molecular dynamics studies [8] show
that the anions are strongly bound to the hydroxyl group on the
cellulose surface, creating a negative electrical charge that weakens
the hydrogen bonds between the cellulose chains.
b) In second place, the separation of the cellulose chains is
promoted by the intercalation of the cations that are attracted by
the anions because of their opposite charge
Experimental parameters of the cellulose dissolution with IL
Table 1 shows the experimental conditions used in different investigations based on the dissolution of cellulose from ionic liquids [15-24].
Table 1:Summary of the experimental conditions of different research works [15].
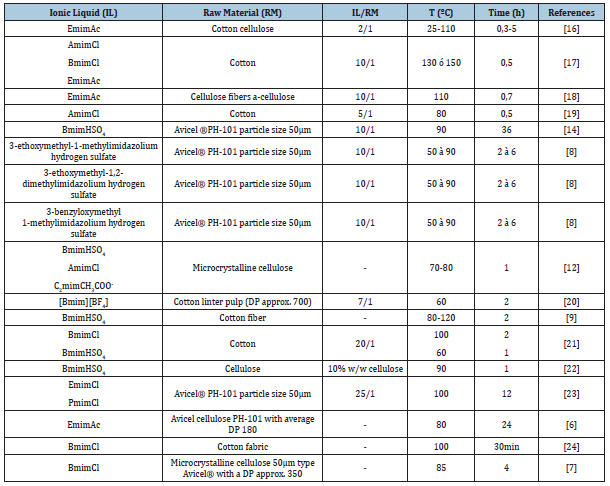
Experimental Methodology
Materials
The treated fabrics have been provided by the company Dagiulio and have been tested as textile waste pre-consumption; 100% cotton fabric, polyester/cotton fabric (30% PES/70%CO), polyester/cotton fabric (52% PES/48% CO) and polyester/cotton fabric (80% PES/20% CO). All fabrics have a grammage of 100g/ m2, are undyed and have been tested in cut pieces of approximately 1x1cm. The solvents used have been Dimethyl Sulfoxide (DMSO) and 1-Allyl-3-Methylimidazole Chloride (AmimCl) provided by Sigma Aldrich.
Experimental procedure
The cut textile fragments are mixed with DMSO (35mL) for 2h, at a temperature of 90 ºC using a hot plate with stirring (300rpm) (Figure 3). The residue is then filtered and mixed with the AmimCl using a 1/16 (w/w) ratio (textile/AmimCl) for 30min, at a temperature of 90 ºC and stirring at 300rpm. The dissolved textile material is drawn into a syringe and extracted into a coagulation bath (water). The regenerated material obtained is dried in the oven for 1 hour at 50 ºC.
Figure 3:Regenerated material after the wet spinning process.

Regenerated material characterization
Scanning electron microscope (SEM):The surface structure of the regenerated material has been analyzed using the PhenomTM Pure Desktop SEM scanning electron microscope. From the images taken, the type of structure obtained and the part of cellulose that has been dissolved can be observed. Before analyzing these samples under the microscope, they are covered with gold (Au) using the SC7620 sputtering metallizer.
Infrared spectroscopy analysis (ATR-FTIR):The chemical structure and composition of the regenerated material has been evaluated by Fourier Transform Infrared Spectroscopy (ATR-FTIR) analysis. Data on the changes and similarities in the molecular bonds of the samples are obtained, in order to detect if there have been changes in the chemical structure of the treated textile. The NicoletTM iSTM10 equipment is used, taking scans in the range of 600-4000cm-1 and with a sample scanning time of 64 scans.
Thermogravimetric analysis (TGA):Thermogravimetric analysis (TGA) has been used to evaluate the thermal stability of the regenerated material and is carried out using the TGA2 STARe System Mettler Toledo equipment. Approximately 5-10mg of the sample were added to a ceramic crucible and heated under inert conditions (N2). The samples were heated in the temperature range of 30-550 ºC at a rate of 10 ºC/minute.
Results and Discussion
Entry of the ionic liquid inside the textile fabric
In order to carry out the dissolution of the cellulose it is necessary to use a solvent that produces fiber’s inflation and acts as a transfer mechanism so that the AmimCl is transported. If water is used, the interactions between AmimCl and cellulose are interrupted. Cellulose and water compete for AmimCl, having preferential interactions between AmimCl and water. Water is a protic polar solvent, therefore forms hydrogen bonds with AmimCl. Contrary to water, DMSO helps to dissolve cellulose, since it allows the inflation of the textile fabric structure. DMSO is an aprotic solvent and does not bond with AmimCl through hydrogen bonds, so the interaction between cellulose and AmimCl is not impeded.
Scanning electron microscope (SEM)
Figure 4:SEM images for the treated samples of (a,b) 100% CO fabric, (c) 30 PES/70 CO fabric, (d) 52 PES/48 CO fabric and (e) 80 PES/20 CO fabric.
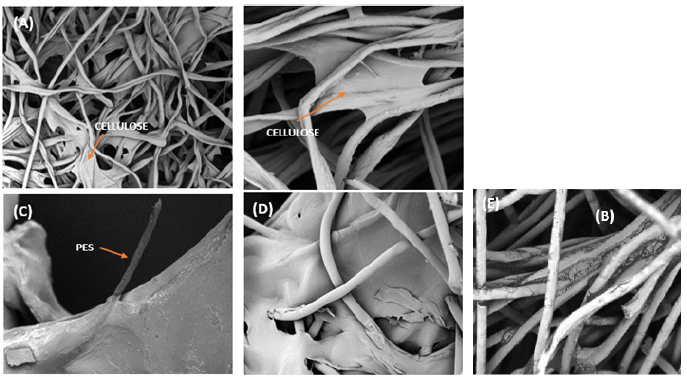
In all the images of (Figure 4), the presence of fragments of regenerated cellulose material that has been dissolved are observed. In the case of 100% cotton treated fabrics, a kind of material composed of cotton fiber and regenerated cellulose material is obtained (Figures 4a & 4b). In the same way for the treated mixtures of PES/CO (Figures 4c-4e), a material composed of the polyester fiber covered by the dissolved cellulose material is obtained. As shown in Figures 4c-4e, the morphology of the polyester fiber has not changed after the applied chemical treatment. Due to stretching, alignment and orientation are not applied to the material in the wet spinning process, irregular and amorphous morphologies are observed in Figure 4.
Infrared spectroscopy analysis (ATR-FTIR)
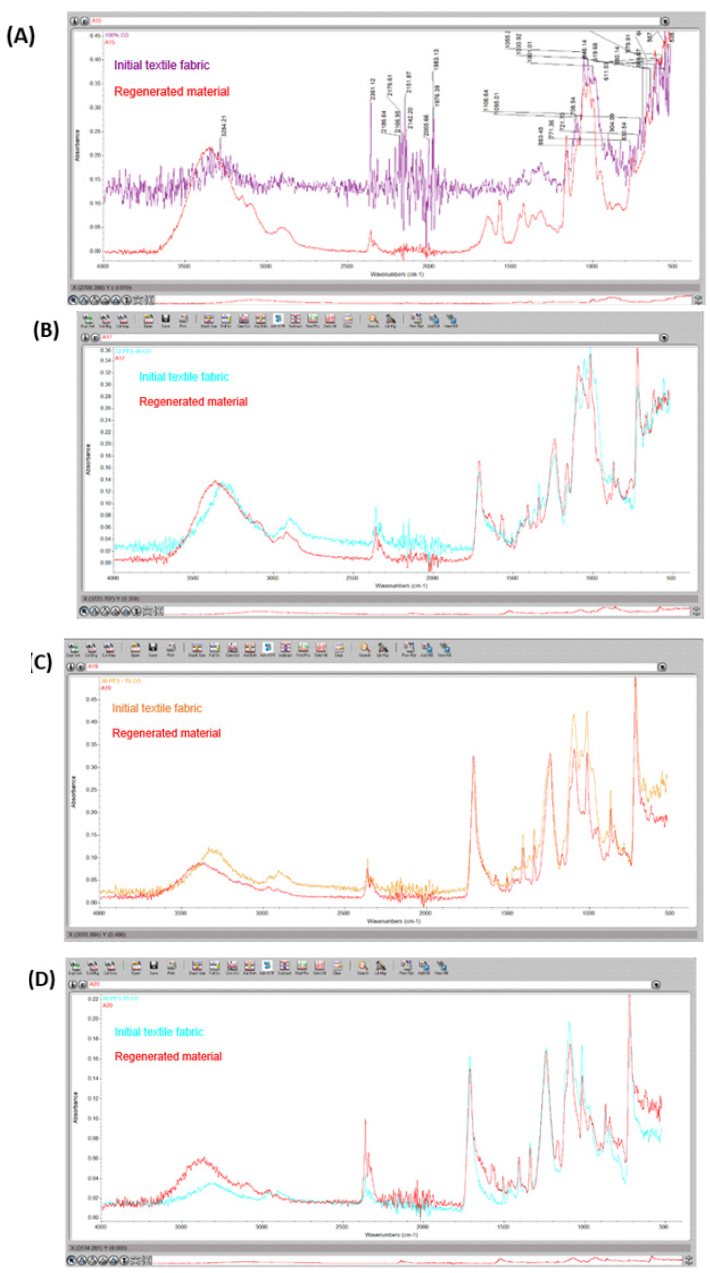
To evaluate the changes that may have occurred in the chemical structure of the treated textile fabric samples, they are analyzed using attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR). Figure 5 shows the spectrum of AmimCl, where the N-H bonds are found at the frequency of approximately 3100cm-1. The area where the imidazoline ring is detected (confirmation peak) is in the right band at a wave number of 1,500cm-1. In the spectra of the treated textile fabrics, superimposed signals of the O-H and N-H groups are observed, since, from the mathematical point of view, spectroscopy is based on the principle of superposition. Therefore, to characterize the textile fabric samples, the results are contrasted with the confirmation peak corresponding to the heterocycle. Figure 6 compares the spectrum of the initial textile fabric to the spectrum of the regenerated material obtained. It is shown that there is no presence of the imidazoline ring. In all the graphs of (Figure 6) it is shown how the spectrum of the initial textile fabric and the spectrum of the regenerated material are practically identical, therefore, no changes in the functional groups and in the chemical structure of the textile sample are detected. The fact that in all cases a greater intensity is observed on the band corresponding to the -OH groups should be taken into consideration. Therefore, it can be assumed that AmimCl breaks the intermolecular bonds and releases the -OH groups outwards, thus having more accessible groups.
Figure 5:FTIR spectrum of the AmimCl ionic liquid.
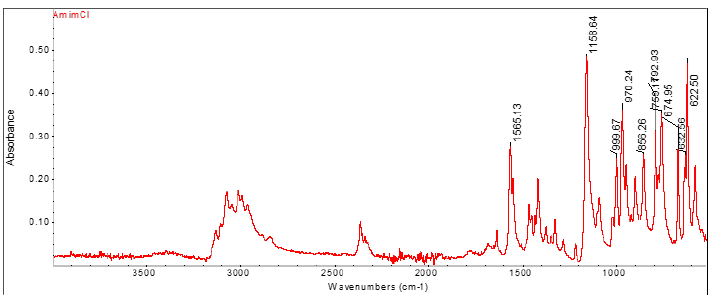
Figure 6:FTIR spectra of the initial and treated textile fabrics of (a) 100% CO, (b) 52% PES/48% CO, (c) 30% PES/70% CO and (d) 80% PES/20% CO.
Thermogravimetric analysis (TGA)
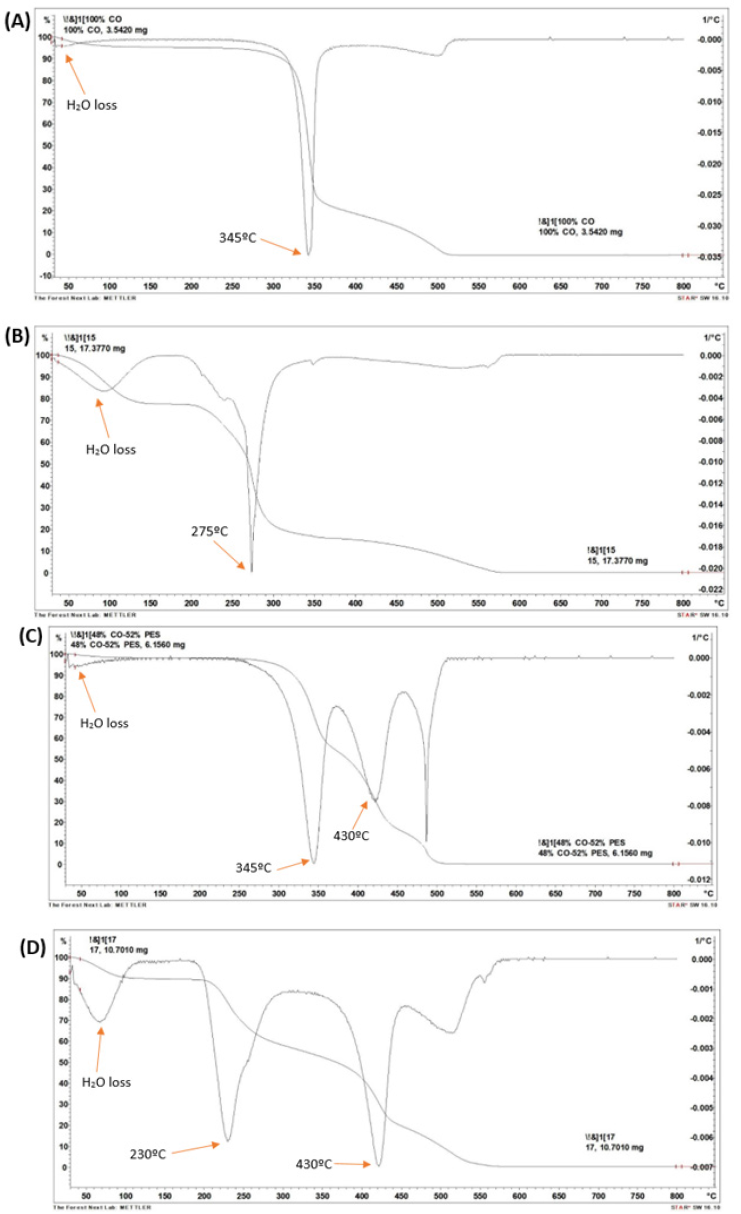
The thermal stability of the regenerated material has been evaluated by thermogravimetric analysis. In the case of the 100% cotton fabric (Figure 7a), a slight weight loss is observed at a temperature below 100 ºC, which corresponds to the evaporation of the water that is on the surface of the sample. Figure 7b shows that once the textile sample is treated, the peak referring to water loss is much more pronounced. This means that the regenerated material has a more disordered (amorphous) structure because it absorbs much more water. At a temperature of 345 ºC, the greatest mass loss occurs for the 100% CO textile fabric (Figure 7a). However, for the treated sample (Figure 7b), it begins to degrade at a lower temperature, approximately 275 °C. Regarding the samples composed of polyester/cotton, a decomposition profile in two steps is observed, the initial step corresponds to the decomposition of cotton and the second to the polyester part. The third peak (T=485 ºC) observed in Figure 7c shows some treatment that may have been applied to the textile fabric, whether it is a heat setting of the polyester or another type of finish on the fabric. Figure 7d also shows that the textile fabrics, once treated, have a more disordered structure, since there is greater water absorption. Figure 7c shows that at temperatures of 345 °C and 430 °C, decomposition of cotton and polyester occurs, respectively. Once this fabric is treated (Figure 7d) only the temperature referring to the cellulosic part reduces to a value of 230 ºC, keeping the polyester area at 430 ºC.
Figure 7:TGA and DTGA curve of textile fabrics (a) 100% CO, (b) 100% regenerated CO, (c) 52% PES/48% CO and (d) 52% PES/48% CO regenerated.
Conclusion
With the elimination of the water in the treatment, the entry of the ionic liquid into the material is achieved, dissolving the textile material and subsequently regenerating the fiber by a simulation of the wet spinning process. The transport medium of the ionic liquid and the solvent used to inflate the fiber has been DMSO. The ratio is the variable that most affects the process, since the chemical mechanism that controls the system is diffusion. It is the ratio that controls the transfer of mass into the textile fabric. With a minimum ratio of 1/16 (w/w) (textile fabric/IL), it is possible to dissolve the raw material. The increase of temperature promotes an increase in the ionic liquid mobility, which helps with the entry of AmimCl into the textile fabric. A temperature of 90 ºC is sufficient to achieve the dissolution of the raw material. With a time of 2h 30min for 100% CO fabrics, the generation of a mass is achieved, which can be extruded through a wet spinning process and for PES/CO fabrics a time of approximately 3h is required. From the SEM images of the treated PES/CO samples, it can be assumed that the recovery of the two fibers is achieved, leading to a new type of fiber. In all the images, the presence of fragments of regenerated cellulose material that has been dissolved is observed. Through the ATR-FTIR analysis it is shown that there are no changes in the chemical structure of the substrate after treatment with AmimCl, since there are no changes in the functional groups. With the thermogravimetric analysis, it is confirmed that the regenerated material has a more amorphous structure, since there is a greater presence of absorbed water. In addition, having a more disordered structure leads to a decrease in the decomposition temperature of the cellulosic material (there are fewer interactions). When it comes to the polyester part, it remains unaltered in its decomposition temperature and therefore, the treatment carried out only affects the molecular interactions of the cotton fiber.
Regarding the effect between the ionic liquid and the cellulose, it is concluded that when the AmimCl interacts with the cellulose, it breaks the intermolecular hydrogen bonds, releasing the -OH groups outwards, obtaining a more amorphous structure when the material is regenerated. With the results obtained, it can be confirmed that from this process it has been possible to obtain a composite material on which the stretching conditions, extrusion speed, orientation... can be controlled in order to obtain better mechanical properties. In conclusion, by using ionic liquids for a cotton/polyester fabric recycling process, the possibility of producing cellulose-based fibers has been demonstrated, achieving the recovery of the two polymers. Direct dissolution and subsequent coagulation in water makes scaling up this process very interesting and therefore, it is a very promising technology with great potential for industrialization. However, the biggest problem facing this process is the recovery and reuse of ionic liquids. For this reason, this is a field to investigate in order to find a solution that requires low energy and that recovers the ionic liquid with high effectiveness.
References
- Carrera Gallissà E (2017) The sustainability challenges of the textile sector. Revista de Química e Industria Textile 220: 20-32.
- Retos y oportunidades en la gestión de los residuos textiles.
- Harmsen P, Scheffer M, Bos H (2021) Textiles for circular fashion: The logic behind recycling options. Sustainability 13(17): 9714.
- Swatloski RP, Spear SK, Holbrey JD, Rogers RD (2002) Dissolution of cellulose with ionic liquids. J Am Chem Soc 124(18): 4974-4975.
- Mohd N, Draman SFS, Salleh MSN, Yusof NB (2017) Dissolution of cellulose in ionic liquid: A review. AIP Conf Proc 1809(1).
- Liu W, Budtova T (2012) Ionic liquid: A powerful solvent for homogeneous starch-cellulose mixing and making films with tuned morphology. Polymer 53(25): 5779-5787.
- Mahmoudian S, Wahit MU, Ismail AF, Yussuf AA (2012) Preparation of regenerated cellulose/montmorillonite nanocomposite films via ionic liquids. Carbohydr Polym 88(4): 1251-1257.
- Grzaabka-Zasadzinska A, Andrzej S, Borysiak S (2019) The influence of the cation type of ionic liquid on the production of nanocrystalline cellulose and mechanical properties of chitosan-based biocomposites. Cellulose 26: 4827-4840.
- Huang J, Hou S, Chen R (2019) Ionic liquid-assisted fabrication of nanocellulose from cotton linter by high pressure homogenization. BioResources 14(4): 7805-7820.
- Sunasee R, Hemraz UD (2018) Synthetic strategies for the fabrication of cationic surface-modified cellulose nanocrystals. Fibers 6(1): 1-19.
- Casas A (2013) dissolution of pinus radiata and eucalyptus globulus wood in ionic liquids based on the 1-alkyl-3-methylimidazolium cation and regeneration of cellulose and lignin. Complutense University of Madrid Faculty of Chemical Sciences, Department of Chemical Engineering, Spain.
- Feng L, Chen ZI (2008) Research progress on dissolution and functional modification of cellulose in ionic liquids. J Mol Liq 142 (1-3): 1-5.
- Zhang J, Wu J, Yu J, Zhang X, He J, et al. (2017) Application of ionic liquids for dissolving cellulose and fabricating cellulose-based materials: State of the art and future trends. Materials Chemistry Frontiers 1(7): 1273-1290.
- Grzabka-Zasadzin A, Amietszajew T, Borysiak S (2017) Thermal and mechanical properties of chitosan nanocomposites with cellulose modified in ionic liquids. J Therm Anal Calorim 130: 143-154.
- Montalbán MG (2005) Properties of ionic liquids and their application to the synthesis of silk fibroin nanoparticles.
- Auxenfans T, Buchoux S, Djellab K, Avondo C, Husson E, et al. (2012) Mild pretreatment and enzymatic saccharification of cellulose with recycled ionic liquids towards one-batch process. Carbohydr Polym 90(2): 805-813.
- Haykir NI, Bahcegul E, Bicak N, Bakir U (2013) Pretreatment of cotton stalk with ionic liquids including 2-hydroxy ethyl ammonium formate to enhance biomass digestibility. Ind Crops Prod 41(1): 430-436.
- Husson E, Buchoux B, Avondo C, Cailleu D, Djellab K, et al. (2011) Enzymatic hydrolysis of ionic liquid-pretreated celluloses: Contribution of CP-MAS 13C NMR and SEM. Bioresour Technol 102(15): 7335-7342.
- Zhang H, Wu J, Zhang J, He J (2005) 1-allyl-3-methylimidazolium chloride room temperature ionic liquid: A new and powerful non derivatizing solvent for cellulose. Macromolecules 38(20): 8272-8277.
- Song X, Zhou L, Ding B, Cui X, Duan Y, et al. (2018) Simultaneous improvement of thermal stability and redispersibility of cellulose nanocrystals by using ionic liquids. Carbohydr Polym 186: 252-259.
- Jordan JH, Easson MW, Condon BD (2020) Cellulose hydrolysis using ionic liquids and inorganic acids under dilute conditions: morphological comparison of nanocellulose. RSC Adv 10(65): 39413-39424.
- Xiao YT, Chin WL, Abd Hamid SB (2015) Facile preparation of highly crystalline nanocellulose by using ionic liquid. Adv Mater Res 1087: 106-110.
- Babicka M, Woźniak M, Dwiecki K, Borysiak S, Ratajczak I 2020) Preparation of nanocellulose using ionic liquids: 1-propyl-3-methylimidazolium chloride and 1-ethyl-3-methylimidazolium chloride. Molecules 25(7): 1544.
- Shibata M, Teramoto N, Nakamura T, Saitoh Y (2013) All-cellulose and all-wood composites by partial dissolution of cotton fabric and wood in ionic liquid. Carbohydr Polym 98(2): 1532-1539.
© 2022 Lis Arias MJ. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






