- Submissions

Full Text
Polymer Science: Peer Review Journal
Correlation Between Swelling and Elasticity of Graphene Oxide-Polyacrylamide Composites
Büşra Osma1, Önder Pekcan2 and Gülşen Akın Evingür3*
1Department of Naval Architecture and Mechanical Engineering, Faculty of Engineering, Pîrî Reis University, Turkey
2Faculty of Engineering and Natural Sciences, Kadir Has University, Turkey
3Department of Industrial Engineering, Faculty of Engineering, Pîrî Reis University, Turkey
*Corresponding author: Gülşen Akın Evingür, Department of Industrial Engineering, Faculty of Engineering, Pîrî Reis University, Tuzla, Istanbul, Turkey
Submission: August 12, 2022;Published: September 06, 2022

ISSN: 2770-6613 Volume4 Issue2
Abstract
Composites of Graphene Oxide (GO) and Polyacrylamide (PAAm) have been proposed as promising biomaterials for biomedicine and tissue engineering. The aim of this work is to investigate the relationship between swelling and mechanical properties of these composites and demonstrate the role of Graphene Oxide (GO) on the swelling and elasticity of the composites. Swelling experiments were performed in distilled water at 30 °C, monitoring the steady-state fluorescence intensity in real time, using Py in the GO-PAAm composites as a fluorescence probe. The diffusion coefficients, Dsc and the cooperative diffusion coefficients Dem were calculated using Fick’s model and Li-Tanaka equations, respectively. The mechanical properties of the composites were investigated by the compression test before and after the swelling process. The GO content dependence of the shear modulus of the composites due to the volume phase transition was measured at 30 °C. After the swelling process, the shear modulus of GOPAAm composites decreases with the increase of GO content above 8μl GO, indicating a critical value. An increase in GO content leads to a decrease in swelling capacity due to more cross-links and cross-link densities in the composites.
Keywords: Graphene oxide; Polyacrylamide; Swelling; Elasticity; Composite
Abbreviations: GO: Graphene Oxide; PAAm: Polyacrylamide; SEM: Scanning Electron Microscopy; PVA: Polyvinyl Alcohol
Introduction
Graphene Oxide (GO) is a lightweight material that has very high strength and thermal stability [1]. It is a two-dimensional carbon material with a thickness of about one atom [2]. Therefore, it is popular material to use in biotechnology, medicine [3] and tissue engineering [4]. In addition, it is an effective filler for improving the electrical, mechanical, and thermal properties of composite materials [2]. The mechanical and thermal properties of Poly (Acrylamide) (PAAm) hydrogels have been modified by the addition of GO [2]. N, N-methylenebisacrylamide (BIS) crosslinks PAAm hydrogels but has several disadvantages such as weakness and brittleness. When GO is added to a hydrogel containing BIS, toughness and tensile strength improve. The content of GO and BIS affects the thermal and mechanical properties of these hydrogels. In addition, GO-BIS gels were observed to have a lower equilibrium swelling ratio than the BIS gels [2]. GO/Polyvinyl Alcohol (PVA) composite film is prepared to observe mechanical strength [5]. When the content of GO is increased, the tensile strength of the composite film initially increases rapidly compared to the pure PVA composite film. The optimum mechanical performance of the film is achieved at a content of 20% of GO by increasing the tensile strength by more than 500% [5]. The pristine GO, silane-functionalized GO (I-GO) and PVI-grafted GO (PVI-g-GO) nanosheets were used to prepare epoxy resin composite to investigate their mechanical and thermal properties [6]. Nanocomposites containing 0.25wt % PVI-g-GO exhibited 59.6% and 45.5% higher tensile strength and modulus, respectively, compared to normal epoxy resin. The result of Scanning Electron Microscopy (SEM) shows a tough structure with a rough surface, which is confirmed by the developed tensile strength [6]. Extrusion-based 3D-printing was used to produce Graphene Oxide (GO)-reinforced HA/gelatin composite scaffold [7]. In this research, mechanical properties of HA/gelation composite were reinforced by GO. Especially, %15 increase of compressive strength and %22 increase of flexural strength were observed addition to %0.5 GO content. According to these experimental results, GO is potential material to produce composite bone scaffolds and develop mechanical properties for application of bone tissue engineering [7].
In this study, the mechanical and swelling behaviour of GO-PAAm composites and the correlation between these two properties are investigated. The fluorescence method was used to observe the swelling kinetics of GO-PAAm composites. The swelling behaviour of the composites was modelled using Fick’s model and the Li-Tanaka equation. On the other hand, the behaviour of the mechanical properties was determined by compressive technique at 30 °C. The composites were examined before and after swelling in water for mechanical measurements. The mechanical behaviour was modelled by the theory of rubber elasticity. It was found that both the swelling behaviour in water and the mechanical properties of the composites were combined for the estimation of physical parameters depending on GO.
Experiment
Preparation of composites
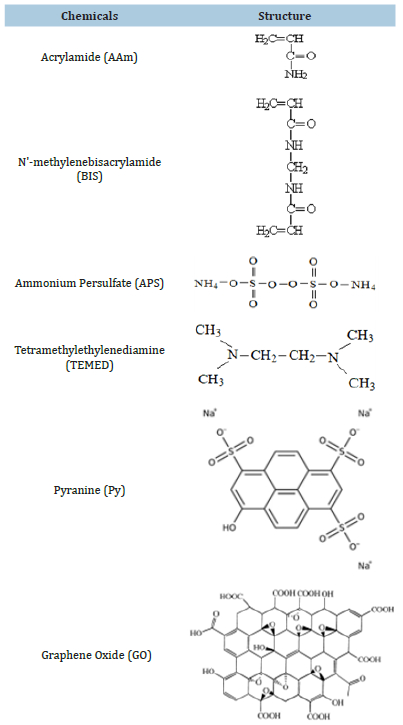
GO content (Graphene Oxide, Graphenea) in a concentration 0, 8, 20, 30, 40 and 50μl and 2M AAm (acrylamide, Merck) were used to prepare composites at room temperature. The concentration of GO obtained from Graphenea is 4mg/ml. The purity of GO is over 95% (wt). 1.42g AAm, 0.02g N’-methylenebisacrylamide (BIS, Merck), 0.016g ammonium persulfate (APS, Merck) and 6μl Tetramethylethylenediamine (TEMED, Merck) were dissolved as a homogeneous solution in 10ml distilled water (pH 6.5) at 200rpm for 15min. GO is added to the solution before mixing TEMED. Pyranine (Py) in the GO-PAAm composites was used as a fluorescent probe. Py is a pyrene derivative with three SO3- groups, so Py can be incorporated into the gels by coulombic attractive forces. GO-PAAm composites were prepared by the method of radical crosslinking copolymerization. Immediately before the addition of TEMED, the sample was deoxidized with bubbling nitrogen for 10 minutes. The solution of 10ml was poured into the injector for swelling and mechanical experiments. The composites were cut from the syringes at room temperature in the form of discs with a diameter of 10mm and a thickness of 4mm [8]. Chemicals are given in Figure 1.
Figure 1:The chemical structures of Acrylamide (AAm) as monomer, N’-methylenebisacrylamide (BIS) as crosslinker, Ammonium Persulfate (APS) as initiator, Tetramethylethylenediamine (TEMED) as accelerator, Pyranine (Py) as fluorescence probe as and Graphene Oxide (GO) as nanoparticle, respectively.
Optical measurements
A spectrometer model LS-55 from Perkin Elmer was used to measure the fluorescence intensity of Pyranine (Py) during the swelling experiments. The spectral band was kept at 5nm while the measurement was performed at 900nm. The disc-shaped composites were in a 1x1x4.5cm3 square quartz cell with stainless steel wire. The square quartz cell was filled with distilled water to determine the swelling behaviour of the composite. The excitation intensity chosen for the in-situ fluorescence experiments was 340nm. The scattered light intensity, Isc and emission light intensity Iem of the pyranine was observed as a function of the swelling time at 340nm, and 427nm to show the change in gel structure. Water diffusion is directly proportional to the emission light intensity, Iem and the intensity of the scattered light, Isc [8]. Thus, when water diffusion increased, Isc and Iem increased due to the increase in structural heterogeneity of the composite [9].
Mechanical measurements
An Instron 3340 testing machine with a loading capacity of 500N was used for the pressure measurements. The different amounts of GO doped composites were prepared in an injector with a diameter of 10mm. They were then cut into 4mm thick slices to use two samples each before and after swelling. The diameter of each slice was measured at least three times with a calibrated digital compass (resolution: 0.01mm). Uniaxial compression tests were performed with an Instron 3340 testing machine for different GO contents of the composites. Before swelling, the pressure measurements were taken at a probe size of 10cm, a speed of 0.1mm/min and an over deformation ratio of 40% at 30 °C. Prior to swelling, the pressure expansion (mm) curves and the pressure load F(N) were measured for 0, 8, 20, 30, 40 and 50μl of the composites using GO at 30 °C. The composites were placed in distilled water to achieve swelling equilibrium at 30 °C and to measure compression for the swelling process. The distilled water was changed at least twice. The samples were kept at room temperature for 1 week. This process is called final washing to achieve swelling equilibrium and to remove unreacted repeated units from the composites. Compression measurements after swelling were performed using the same method as described above.
Results and Discussion
Diffusion and swelling
The fluorescence spectra of pyranine in the composites were observed during swelling at 20 and 50 minutes for 50μl content of GO doped composites, as shown in Figure 2a. Iem and Isc increased when water uptake was increased during swelling. In Figure 1, the fluorescence spectrum of swelling of 20 and 50μl of doped PAAm composite at 100min and 30 °C is shown by different lines. As can be seen in Figure 2b, Isc and Iem increase with increasing GO content in the distilled water, resulting in high shielding and more lattice heterogeneities [10]. The change in scattered light intensities Isc as a function of swelling time of the GO-PAAm composites is shown in Figure 3a during the swelling process for 8 and 40μl GO at 30 °C. Assuming that Isc is proportional to the number of water molecules entering the composite, the behaviour of the intensity curves in Figure 3a suggests that the water molecules are absorbed by the composite network and cause more scattering. This assumption can be explained by “frozen blobs”. It is well known that a “frozen blob” appears where two junctions are positioned on adjacent lattice sites [11]. Here we can assume that the penetration of water into the composite is lower in gels with high GO content than in gels with low GO content, which is due to lower solubility and/or high shielding. Based on this picture, the diffusion process can be described using Fick’s diffusion model. In this case, the GO-PAAm composites are considered as thin plates and the solution of the equation is given by the following equation [12].
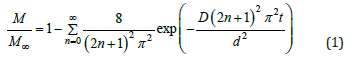
Figure 2:Fluorescence spectra of pyranine in the composites prepared with (a) 50μl of GO during the swelling process at 30 °C for 20 and 50min, and (b) 20 and 50μl of GO at 100min. and 30 °C, respectively.
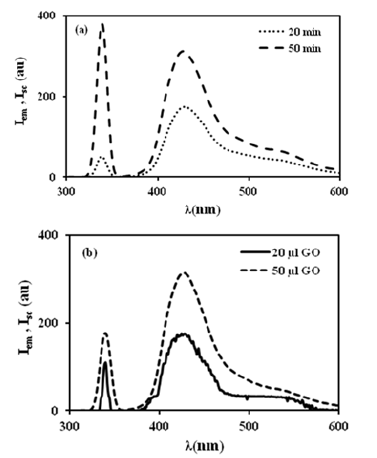
Figure 3a:Scattered light intensities, Isc versus time, t during swelling process for 8, and 40μl of GO content

where d is the thickness of the sample and M and M∞ are the masses of water absorbed at time t and ∞ respectively. Then the logarithmic form of Eq.1 for n=0 can be given as follows:
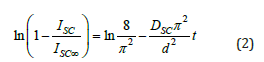
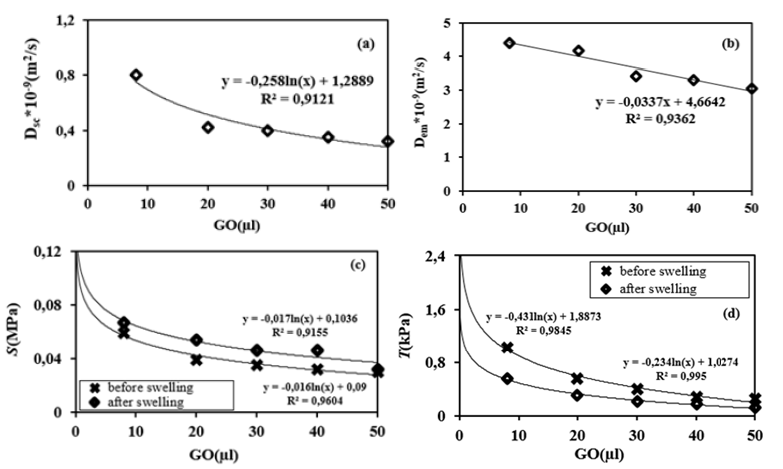
where the scattered light intensity Isc is proportional to the mass of water absorbed at time t. Dsc is the diffusion coefficient from the scattered light intensity and d is the thickness. In Fick’s diffusion model, the curve in Figure 2a should be linear with respect to Equation 2 and have a slope from which the diffusion coefficient Dsc can be calculated. The curves in the form of equation 2 for 8μl GO in the composites are shown in Figure 3b. It was observed that the slope of the regression lines corresponding to the diffusion coefficient is shown in Figure 4a. Here it can be seen that high Dsc values are observed for composites with low GO content, which is expected due to low shielding and/or high solubility. With increasing GO content, the Dsc values decrease due to the low accessibility of the composite for water molecules.
Figure 3b:logarithmic form of the data in Figure 2(a) by using Eq. 2 for 8μl of GO content at 30 °C, respectively.
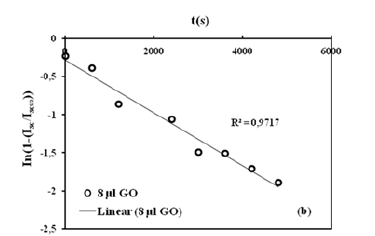
Figure 4a:The fluorescence intensities, Iem versus time, t during swelling process for 8, and 40μl of GO content.
The fluorescence intensity, Iem versus swelling time is shown in Figure 4a for 8 and 40μl of GO doped composites at 30 °C, respectively. It can be seen in Figure 4a that Iem increases with increasing swelling time, indicating that the composite becomes more transparent with increasing water uptake and more Py molecules leak out, resulting in higher emission. A low Iem intensity at a high GO content indicates that more lattice heterogeneities are present during water uptake of the composite. Therefore, the Iem intensity in the composite is low at a high GO content. During the swelling process, when water molecules enter the gel, more structural complexities are formed, so that Iem in a gel with a high GO content has lower values than in a gel with a low GO content. With this behaviour, equation 3 can be written in terms of Iem. The curves of this typical solvent uptake correspond to the Li-Tanaka equation [8],
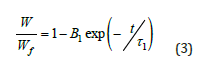
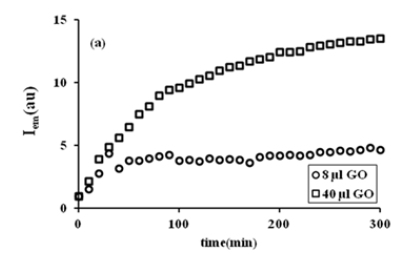
where, τ1 is the time constant, measured by the fluorescence technique, B1 is related to the ratio of the shear modulus, μ and longitudinal osmotic modulus, M and t is time. The following relationship was derived from Equation 3 to fit the logarithmic form of the data in Figure 4a.
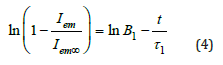
Where τ1 is the time constant, and values are used to create the linear regression of the curves using equation (4). The linear regression of the curves is shown in Figure 4b. R graph are based on the method described by Li and Tanaka. Considering these graphs, R values were obtained from B1 and R graph, then α1 was obtained from α1 and R graph, respectively. Then the following equation (5)
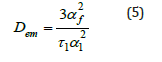
Figure 4b:The linear regression of the data in Figure 3(a) fitted to the Li-Tanaka Model (Eq. 4) for 8μl of GO content at 30 °C.
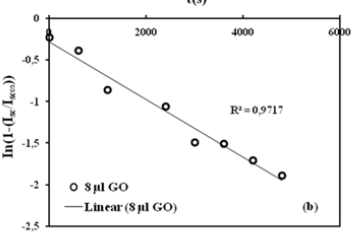
was used to determine the cooperative diffusion coefficients Dem of these disc-shaped composites, and they are about 10-9m2/ s, as shown in Figure 4b. It was observed that composites with a high content of GO swell much slower, resulting in smaller Dem coefficients for all measurements at a given temperature. The Dem values decrease with increasing GO content, which is due to the increasing stiffness of the gel system, i.e., the low accessibility of the composite due to low solubility.
Elasticity
From classical thermodynamics the equation of state for rubber elasticity may be expressed as [13],
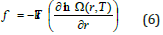
where, k is the Boltzmann constant, r is a certain end-toend distance, and Ω(r,T ) is the probability that the polymer chain with an end-to-end distance r at temperature T will adopt a certain conformation. After evaluation of eq. (6), the statistical thermodynamic equation of state for rubber elasticity is obtained below
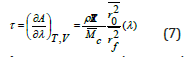
Here, τ is the shear stress per unit area, ρ is the density of the polymer, Mc is the number average molecular weight between crosslinks, and λ is the extension/compression ratio. Extension/ compression ratio, λ changes by different theories [13]. The quantity 2 2 0 f r r is the ratio of the end-to-end distance in a real network versus the end-to-end distance of isolated chains. Network imperfections such as cycles, chain entanglements, and chain ends are not considered. The loaded composites were tested for elastic behaviour using uniaxial compression tests for all GO contents at 30 °C. Figure 5a shows the force- compression curves before and after swelling for 40μl of the contents of GO at 30 °C. From the rubber elasticity [14- 16] the change in the length of the compressed sample, ΔL, is given in equation (8)
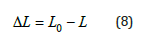
Figure 5a:Force versus compression.
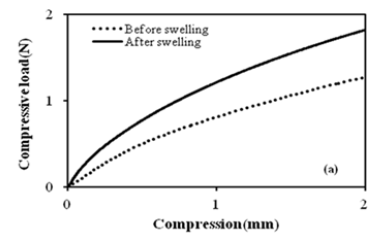
Where is the L0 original length of the specimen and L is the deformed length of the specimen along the vertical axis. The compressive stress was calculated according to the following equation (9)
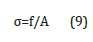
where f and A are the applied force and the initial crosssectional area of the specimen perpendicular to the direction of loading, respectively. The curves for compressive stress and strain before and after swelling for 40μl of the contents of GO at 30 °C are shown in Figure 5b. A stress and strain curve is drawn based on the following linear dependence with the values of load and displacement recorded in the experiment. The shear modulus, S is calculated according to the following equation (10):
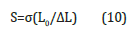
Figure 5b:Force versus compression.
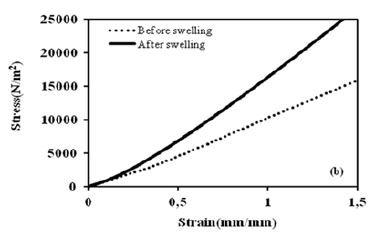
Where ΔL is transverse displacement. The shear modulus, S is determined by the slope of the stress-strain curves within the linear elastic regime of less than 5% strain (see Figure 5b). The shear modulus as a function of different GO contents from mechanical measurements is shown in Figure 5. The shear modulus of GO - PAAm composites before and after swelling decrease with the increase of GO content above 8μl GO. It is also known that the swelling properties of composites mainly affect the shear modulus of the composites [1,14-16]. Here it can be seen that the composite has a lower G-value before swelling than after swelling, indicating higher toughness, i.e., high stiffness. A comparison of Figures 6a- 6d shows that the diffusion coefficients D and the shear modulus S behave similarly as a function of the GO content, indicating that a higher GO content leads to tougher composite gels. In other words, the content of GO could act as a multifunctional crosslinker to form more compounds in the GO-PAAm composite and increase the crosslink density, leading to a decrease in swelling capacity. The toughness, T of the composite after swelling is found to be lower than the toughness after swelling between 8 and 50μl GO in Figure 6d [14]. It is obvious that toughness of composite gel with water must be less than the gel without water. It is also well known that the swelling properties of composite gels mainly affect the shear modulus and toughness of the composite gels. Because basically composite assembly based on intermolecular hydrogen bond or other non-covalent interactions was constructed in the presence of the GO and PAAm networks, which further affected swelling performances of the composites [16]. It was deduced that the GO content could act as a multifunctional cross-linker to form more junctions in GO–PAAm composite and increase the crosslink density, leading to the reduction of swelling capacity [2].
Figure 6:Diffusion coefficients, Dsc and Dem calculated from the data of (a) scattered light intensity, (b) the fluorescence intensity versus GO content in the composites for swelling process, (c) shear modulus and (d) toughness of GO-PAAm composites versus GO content before and after swelling process at 30 °C, respectively.
Conclusion
The swelling and elasticity of graphene (GO) - polyacrylamide (PAAm) composites were investigated using fluorescence and compression techniques, respectively. The Fick’s and Li Tanaka models were used to calculate the diffusion coefficients Dsc and Dem. The swelling of the composites was investigated as a function of GO. It was found that the diffusion coefficient and the cooperative diffusion coefficient decreased with increasing GO content. The shear modulus was also decreased before and after the swelling process from the critical value, which is at a content of 8μl of GO. After reaching the highest shear modulus at 8μl of GO, the shear modulus decreased with increasing GO content. Three contributions were identified, consisting of the degradation of physical cross-links, relaxation common to elastomers and swelling-induced relaxation, to produce the overall stress relaxation of GO -PAAm composites [1,14-16].
References
- Huang Y, Zeng M, Ren J, Wang J, Fan L, et al. (2012) Preparation and swelling properties of graphene oxide/poly (acrylic acid-co-acrylamide) super-absorbent hydrogel nanocomposites. Colloids Surf A 401: 97-106.
- Shen J, Yan B, Li T, Long Y, Li N, et al. (2012) Study on graphene-oxide-based polyacrylamide composite hydrogels. Composites: Part A 43(9): 1476-1481.
- Vargas C, Simarro R, Reina JA, Bautista LF, Molina MC, et al. (2019) New approach for biological synthesis of reduced graphene oxide. Biochem Eng J 151: 107331.
- Purohit SD, Bhaskar R, Singh H, Yaday I, Gupta MK, et al. (2019) Development of a nanocomposite scaffold of gelatin-alginate-graphene oxide for bone tissue engineering. Int J Biol Macromol 133: 592-602.
- Chengan T, Hao Z, Fang W, Hui Z, Xiaorong Z, et al. (2017) Mechanical properties of graphene oxide/polyvinyl alcohol composite film. Polym & Polym Compos 25(1): 11-16.
- Pour Z S, Ghaemy M (2016) Polymer grafted graphene oxide: For improved dispersion in epoxy resin and enhancement of mechanical properties of nanocomposite. Composites Science and Technology 136: 145-157.
- Lee H, Yoo JM, Ponnusamy NK, Nam SY (2022) 3D-printed hydroxyapatite/gelation bone scaffold reinforced with graphene oxide: Optimized fabrication and mechanical characterization. Ceramics International 48(7): 10155-10163.
- Osma B, Evingür GA, Pekcan Ö (2019) Effect of temperature and graphene oxide on the swelling of PAAm-GO composite gels. ICNFA 146: 1-5.
- Evingür GA, Karsli K, Pekcan Ö (2008) In situ fluorescence study of swelling, sorption and desorption processes in and out of PAAm gels. Macromolecular Symposia 265(1): 100-110.
- Evingür GA, Pekcan Ö (2017) Elastic properties of a swollen polyacrylamide (PAAm) gel doped with various multiwalled carbon nanotube (MWNT) contents. Mech Test 59: 485-490.
- Arda E, Kara S, Pekcan Ö, Evingür GA (2021) Evaluation of the fractal dimension of polyacrylamide during gelation and swelling. Materials Today Communications 26: 101980.
- Evingür GA, Karslı K, Pekcan Ö (2006) Monitoring small molecule diffusion into hydrogels at various temperatures by fluorescence technique. International Journal of Pharmaceutics 326(1-2): 7-12.
- Anseth KS, Bowman CN, Peppas LB (1996) Mechanical properties of hydrogels and their experimental determination. Bio Mater 17(17): 1647-1657.
- Evingür GA, Pekcan Ö (2018) Mechanical properties of graphene oxide-polyacrylamide composites before and after swelling in water. Polym Bull 75: 1431-1439.
- Evingür GA, Sağlam NA, Çimen B, Uysal BÖ, Pekcan Ö (2020) The WS2 dependence on the elasticity and optical band gap energies of swollen PAAm composites. Journal of Composite Materials 55(1): 1-6.
- Evingür GA, Pekcan Ö (2014) Effect of multiwalled carbon nanotube (MWNT) on the behavior of swelling of polyacrylamide–MWNT composites. J Reinf Plast Compos 33(13): 1199-1206.
© 2022 Gülşen Akın Evingür. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






