- Submissions

Full Text
Polymer Science: Peer Review Journal
Gold Nanocomposites Based on 1-Vinyl-1,2,4- Triazole Copolymer with Acrylic Acid
Sargsyan SA1*, Sargsyan AS2, Sargsyan TS2, Khizantsyan KM1, Agajanyan IG1, Gevorgyan KV1 and Margaryan KS2
1National Polytechnic University of Armenia, Armenia
2Yerevan State Medical University after Mkhitar Heratsi, Armenia
*Corresponding author: Sargsyan SA, National Polytechnic University of Armenia, Yerevan, Teryan 105, 0009, Armenia
Submission: March 08, 2022;Published: March 18, 2022

ISSN: 2770-6613 Volume3 Issue2
Abstract
The paper discusses the results of electrosynthesis of metal-polymer nanocomposites of gold and their coating of pure iron and steel electrodes when combining the process of electro polymerization of 1-vinyl- 1.2.4-triazole with acrylic acid during cathodic separation of metals. Using UV, IR and atomic absorption spectroscopies, X-ray phase analysis, as well as thermogravimetric and elemental analyses, the structure and composition of the synthesized nanocomposites and nanocomposite coatings were studied.
Keywords: Electrolysis; Nanocomposites; 1-vinyl-1.2.4-triazole; Gold; Electro polymerization
Introduction
Electrosynthesis and study of the properties of functional polymers and nanomaterials obtained on their basis is the most rapidly developing area of modern chemical science. As noted in [1-5], nanomaterials are used in medicine as antibacterial drugs, systems for targeted delivery of contrast agents and drugs, biosensors, and other biomedical purposes. Nanocomposite materials containing gold nanoparticles have unique properties and their development is promising for medicine, nanophotonic and catalysis [1,2].
Mini Review
The synthesis of new functional thrombo-resistant, non-toxic polymeric materials with gold nanoparticles makes it possible to expand the area of their application, as well as to increase the range of materials used in pharmaceuticals when creating new dosage forms. The properties of nanocomposites substantially depend on both the nature of the stabilizing polymer matrix and the conditions for the nanoparticle’s formation. As a polymer matrix, 1-Vinyl-1.2.4-Triazole (VT) polymers and copolymers can be used, since they are non-toxic (LD50> 3000mg·kg–1) and have high film formation ability, solubility, and biocompatibility [6,7]. In this work, we discuss the results of electrosynthesis of metal-polymer nanocomposites and their coatings on pure iron and steel electrodes when the process of electrochemical (co) polymerization of solid solution with Acrylic Acid (AA) is combined with cathodic separation of metals. During the electrolysis of aqueous or water-ethanol solutions of VT and AА or their mixtures at various ratios in the presence of HAuCl4 and chitosan, nanocomposites, and nanocomposite coatings with a gold content of 1-10wt% are formed only in the presence of a peroxide-type initiator, for example, TBOBA, the potential of electroreduction of which is close to the potentials of the cathodic separation of metals - 0.6-1.2V (c.s.e.). After drying, the formed nanocomposite coatings on the electrodes become insoluble in water and in commonly used organic solvents (dimethylsulfoxide, dimethylformamide, acetonitrile, etc.). Likely, the copolymer is crosslinked upon heating. In the electronic spectra of gold-containing nanocomposites, in contrast to aqueous solutions of the initial copolymers and HAuCl4, plasmon absorption bands appear with a maximum in the region of nanocomposite coatings containing gold, absorption bands appear with a maximum in the region of 517-521nm, which is typical for systems with zero-valent gold.
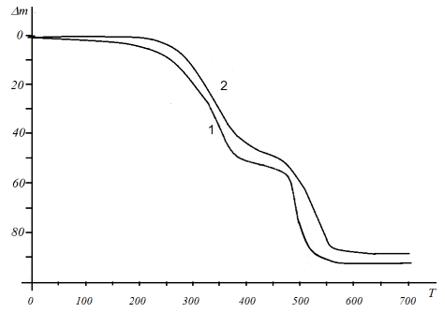
The IR spectra of VT-AA copolymers contain bands corresponding to the frequencies of stretching bending vibrations of the triazole ring 1503, 1434, 1138, 1005, 660cm-1 (C-N, C=N), 1275cm-1 (NN), 3106cm-1 (CH), and a band at 1711cm-1 related to stretching vibrations of the units of the carboxyl group (Figure 1). Analysis of IR spectra shows that the formation of gold-containing polymer nanocomposites leads to insignificant changes in the chemical structure of the copolymer matrix. Thus, the intensities of the absorption bands of the triazole ring, which can act as coordination centers of gold nanoparticles, show a weak shift (by 3-4cm-1) of one band at 1506cm-1, which is characteristic of the stretching vibrations of the ring (C-N and C=N). This shift may indicate the coordination interaction of the triazole ring with the surface atoms of metal nanoparticles. As a result of this work, a nanocomposite of the following structure is formed (Figure 2). The sizes of gold nanoparticles were calculated according. According to the results of transmission electron microscopy (Figure 3a), nanocomposites contain mostly elliptical gold nanoparticles, uniformly distributed in the polymer matrix, having sizes of 2-10nm (Figure 3b). The study of the thermal stability of polymer nanocomposites showed that the first stage of polymer matrix destruction is observed in the temperature range from 260 ºC to 400 ºC and is accompanied by a gradual weight loss of up to 45%, which, likely, refers to the elimination and oxidation of carboxyl (Figure 4).
Figure 1: Electronic absorption spectra of VT-AA copolymer (1), 4.8% (2), 6.1% (3).
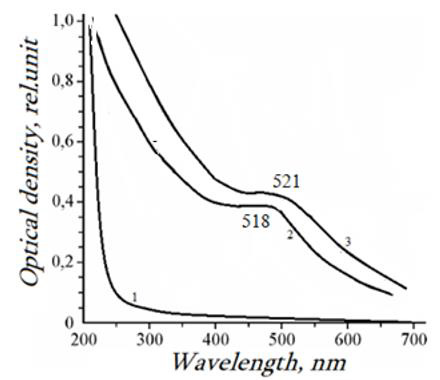
Figure 2: The reaction schematic.
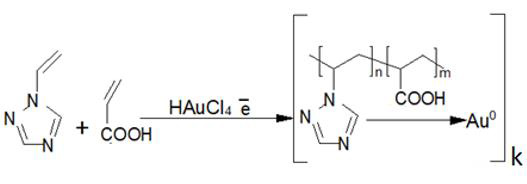
Figure 3: Electron micrograph (a) and distribution diagram (b) gold nanoparticles by size in the VT-AA copolymer matrix.
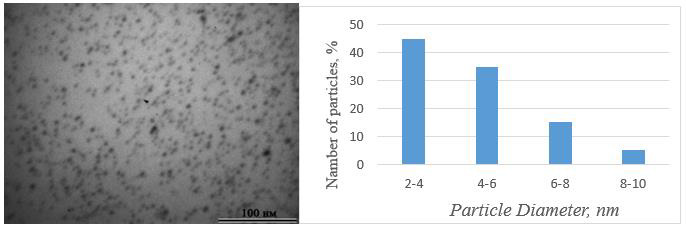
Figure 4: Thermogravimetric curves: 1- copolymer VT-AA, 2 - gold-bearing nanocomposite, - weight loss (mass %), T-temperature (ºC).
Conclusion
The possibility of forming nanocomposites and nanocomposite coatings of gold by the electrochemical method based on VT and AA has been shown, and some properties have been studied.
References
- Pomogailo AD, Rosenberg AS, Uflyand IE (2000) Metal nanoparticles in polymers. Chemistry, Moscow, Russia, p. 672.
- Wang R (2004) The chemistry of nanomaterials. Colloid Polym Sci 283: 234. In: Rao CNR, Müller A, Cheetham AK (Eds.), (2004) WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, p. 741.
- Broz P (2010) Polymer-based nanostructures: Medical applications. Roy Soc Chem, Cambridge, United Kingdom.
- Noroozi M, Zakaria A, Moksin MM, Wahab ZA, Abedini A (2012) Green formation of spherical and dendritic silver nanostructures under microwave irradiation without reducing agent. Int J Mol Sci 13(7): 8086-8096.
- Sargsyan SH, Margaryan KS (2014) Formation of composite polymeric coatings on pure iron. Russian Journal of General Chemistry 84: 550-551.
- Deligoz H, Baykal A, Senel M, Sozeri H, Karaoglu E, et al. (2012) Synthesis and characterization of poly(1-vinyltriazole)-grafted superparamagnetic iron oxide nanoparticles. Synthetic Metals 162(7-8): 590-597.
- Sen U, Bozkurt A, Ata A (2010) Nafion/poly(1-vinyl-1,2,4-triazole) blends as proton conducting membranes for polymer electrolyte membrane fuel cells. Journal of Power Sources 195(23): 7720-7726.
© 2022 Sargsyan SA. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






