- Submissions

Full Text
Polymer Science: Peer Review Journal
Chitosan as Reinforcement for Biopolymers - A Mini Review
Dabertrand M, Audonnet F and de Baynast H*
Université Clermont Auvergne, Clermont Auvergne INP, CNRS, Institut Pascal, F-63000 Clermont-Ferrand, France
*Corresponding author: H de Baynast, Université Clermont Auvergne, Clermont Auvergne INP, CNRS, Institut Pascal, F-63000 Clermont-Ferrand, France
Submission: February 15, 2022;Published: March 09, 2022

ISSN: 2770-6613 Volume3 Issue2
Abstract
It is nowadays urgent to find new packaging solutions that have a lower impact on the environment. Biopolymers (PLA, starch, PBAT) seem interesting to the design of the new packaging generation. These polymers have some drawbacks such as mechanical and barrier properties. Also, it could be interesting to add antimicrobial properties to packaging to increase the shelf life of food products. For those reasons, chitosan seems to be an interesting component to add to a biopolymer matrix. Chitosan is a polysaccharide derived from crustacean shells. Chitosan can be directly added to the matrix via thermomechanical processes, or it can be turned into thermoplastic chitosan with the combined effect of an acid solution, a plasticizer, heat, and shear. The chitosan intramolecular bonds are thus reduced. The second method seems to limit the agglomeration of chitosan in the polymer matrix and increase interfacial adhesion. The composite properties depend on the acid and plasticizer type and rate.
Keywords: Antimicrobial properties; Biopolymer; Chitosan; Composite; Plasticised chitosane
Introduction
Packaging is nowadays one of the main polluting fields, particularly because of plastic packaging [1,2]. Plastic world production was 367 million tons in 2020 [3]. It generates a massive amount of waste, which is not enough valorized [4]. As the field grows, it is urgent to find new packaging solutions that preserve food from spoiling and have a lower environmental impact. For those reasons, biopolymers seem promising to replace oil-based polymers currently used [5,6]. More precisely, biopolymers such as Polylactic Acid (PLA) [7,8], starch [9,10], Polybutylene Succinate (PBS) [11-13], Poly (Butylene Succinate-Co-Butylene Adipate) (PBSA) [12,13], and Polybutylene Adipate Terephthalate (PBAT) [14,15] are widely studied [16,17]. Those polymers can be transformed by thermomechanical processes (injection, extrusion, thermoforming), which is an advantage for industrial production. However, biopolymers also have drawbacks. Mechanical, barrier and optical properties can be weak for some applications. Design composites can be an interesting way to improve polymer’s properties. To produce those structures, reinforcements are dispersed in polymers matrix. Cellulose, silica, clay, and alumina are the main studied reinforcements [18]. Nonetheless, chitosan has proved that it could be an interesting candidate due to its antimicrobial properties [19-23]. This property could increase the shelf life of food products and limit spoilage. Thus, this review aims to present the recent advances in biopolymers reinforcement with chitosan.
Chitosan
Chitosan is a polysaccharide derived from chitin by deacetylation (Figure 1); [24,25]. Chitin is extracted from shellfish skeletons and exoskeletons, mainly from crustacean shells. Seafood industry generates 80.000 tons of waste per year [26]. Consequently, it could be interesting to recover this material. Chitosan is biodegradable, biocompatible, non-toxic, antimicrobial. It is also chemically modifiable, which allows to adapt the properties [21,23]. It is insoluble in water but soluble in acidic conditions. Chitosan is usually defined by its molecular weight and its Deacetylation Degree (DD). Chitosan cannot be transformed by common plastics processes (injection, extrusion, thermoforming) because it is not thermoplastic. The degradation temperature is lower than the melt temperature. This is due to strong intermolecular bonds that prevent the melting, flow, and deformation [27]. Nonetheless, chitosan can be added to polymers as reinforcement by thermomechanical processes.
Figure 1: Chitin deacetylation reaction [24,25].

Raw Chitosan as Reinforcement for Biopolymer
Reinforcement of thermoplastic polymers by chitosan was studied by directly adding chitosan without any treatment. Correlo et al. mixed biopolyesters (PLA, PBS, PBSAT, PBSA, Polycaprolactone (PCL)) with chitosan (25 à 70 %wt.) via twinscrew extrusion and injection [28]. Clusters of chitosan are observed in samples (100-300μmx15-30μm). Chitosan clusters are larger than the initial size of chitosan, which suggests the chitosan agglomeration. Tensile strength reduction was observed and depend on the polymer considered: a diminution of 34 % and 11 % for 50 %wt. of chitosan in PBSA composite and PBAT composite was obtained, respectively. Those results suggest a weak interfacial adhesion. Bonilla et al. obtained similar results by studying PLA/ chitosan (Mw=161kDa; DD=77 %) composites made by twin-screw extrusion and cast extrusion [29]. Chitosan seems well dispersed but obtained films present irregularities and rugosity because of chitosan particles. Matrix and polymer seem immiscible and noncompatible. However, antimicrobial properties were observed for chitosan-based films and allow a reduction of bacterial growth on pork meat. Díez-Pascual et al. have also studied composites with chitosan. Nanofibers were produced by electrospinning and were mixed with PBAT via solvent casting method [30]. Composites were considered efficient from 5 %wt. of chitosan against 4 pathogens (Staphylococcus aureus, Bacillus subtilis, Salmonella enteritidis and Escherichia coli). Other studies have demonstrated the antimicrobial properties of chitosan-based composites (PLA/starch/chitosan, PLA/chitosan, PCL/chitosan) [31-33].
Thermoplastic Chitosan Blend
Chitosan-based composites can present agglomeration and compatibilization issues. Produced Thermoplastic Chitosan (TPC) can be a good method to avoid those problems. Chitosan can be converted into TPC by the combined effect of heat, shear, and the presence of a plasticizer. This method is widely studied in starch’s case (production of Thermoplastic Starch (TPS)) [9,10,34]. Small molecules are located between polymer chains by means of the thermomechanical process (generally twin-screw extrusion or internal mixer). Intermolecular bonds are reduced, and chains mobility is increased [27,35]. Chitosan can consequently melt. TPC production requires two components:
a. An acid solution, which allows the protonation of chitosan: acetic, hydrochloric, and lactic acid can be used [36]. Hydrochloric acid is a strong acid that is efficient but can degrade chitosan at a high rate. Acetic acid is a weak acid. Its action of protonation is less efficient than hydrochloric acid, but it cannot degrade chitosan [36-38]. The protonation reaction by lactic acid is presented in Figure 2. The sterically hindered caused by the acid leads to an increase in the space between molecular chains [36-38].
Figure 2: Chitin deacetylation reaction [24,25].
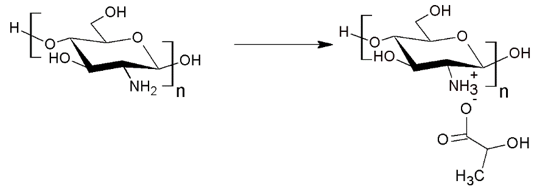
b. A plasticizer, which increases the space between chitosan chains. It strengthens the acid role. Propylene glycol, polyethylene glycol, and polyols (glycerol, xylitol, sorbitol) can be used [35,38,39]. According to the authors, sorbitol gives better thermal and mechanical properties than glycerol and xylitol [35].
Both acid and plasticizer have an impact on chitosan crystal structure [35]. Some studies aim to improve TPS properties by adding Thermoplastic Chitosan (TPC). Deng et al. prepared TPC via casting method and mixed it with TPS by twin-screw extrusion [40]. TPC increases the viscosity of TPS and thus facilitates the processing. Hygroscopy and elongation are lower, and Young Modulus is higher than neat TPS. Mendes et al. studied the same blend and demonstrated that chitosan and starch are well dispersed, as there is no chitosan cluster [41]. Young modulus and elongation are also improved.
Moreover, some published work deals with oil-based polymer and TPS. Matet et al. studied the Polyethylene (PE)/Ethylene-Vinyl Acetate (EVA)/TPC blend, which was produced by extrusion [35]. 90 % of chitosan areas are smaller than 10μm large, which means that the plastification works. The obtained films are yellowish. The Young modulus and elongation are reduced. The same authors in another study demonstrate that PE/chitosan composites have antimicrobial properties [42].
Conclusion
Chitosan is a widely studied polysaccharide because it is a biobased, biodegradable, non-toxic, and antimicrobial component. Chitosan can be added to a polymer matrix by thermomechanical processes. Some of those composites have antimicrobial properties, which can be interesting to increase the shelf life of food products. However, polymer matrix and chitosan are often non-compatible. That can lead to a reduction of polymers properties, such as mechanical properties. Recently, TPC production methods have been studied. TPC was mixed with other polymers to increase properties. A reduction of agglomeration was noted. This is promising for further designs of the next packaging generation.
References
- Jabeen N, Majid I, Nayik GA (2015) Bioplastics and food packaging: A review. Cogent Food & Agriculture 1(1).
- Geyer R, Jambeck JR, Law KL (2017) Production, use, and fate of all plastics ever made. Sci Adv 3(7).
- (2020) Plastics - The facts 2020, Plastics Europe.
- Guillard V, Gaucel S, Fornaciari C, Coussy HA, Buche P, et al. (2018) The next generation of sustainable food packaging to preserve our environment in a circular economy context. Front Nutr.
- Helanto K, Matikainen L, Talja R, Rojas OJ (2019) Biobased polymers for sustainable packaging and biobarriers : A critical review. BioResources 14(2): 4902-4951.
- Mangaraj S, Yadav A, Bal LM, Dash SK, Mahanti NK (2019) Application of biodegradable polymers in food packaging industry: A comprehensive review. J Package Technol Res 3: 77-96.
- Garlotta D (2001) A literature review of poly (lactic acid). Journal of Polymers and the Environment 9: 63-84.
- Farah S, Anderson DG, Langer R (2016) Physical and mechanical properties of PLA, and their functions in widespread applications - A comprehensive review. Advanced Drug Delivery Reviews 107: 367-392.
- Janssen LPBM, Moscicki L (2009) Thermoplastic starch: A green material for various industries, Wiley-VCH, Weinheim, Germany.
- Zhang Y, Rempel C, Liu Q (2014) Thermoplastic starch processing and characteristics - A review. Critical Reviews in Food Science and Nutrition 54(10): 1353-1370.
- Mochane MJ, Magagula SI, Sefadi JS, Mokhena TC (2021) A review on green composites based on natural fiber-reinforced polybutylene succinate (PBS). Polymers 13(8): 1200.
- Xu J, Guo BH (2010) Poly (butylene succinate) and its copolymers: Research, development and industrialization. Biotechnology Journal 5(11): 1149-1163.
- Siracusa V, Lotti N, Munari A, Dalla Rosa M (2015) Poly (butylene succinate) and poly (butylene succinate-co-adipate) for food packaging applications: Gas barrier properties after stressed treatments. Polymer Degradation and Stability 119: 35-45.
- Gan Z, Kuwabara K, Yamamoto M, Abe H, Doi Y (2004) Solid-state structures and thermal properties of aliphatic–aromatic poly (butylene adipate-co-butylene terephthalate) copolyesters. Polymer Degradation and Stability 83(2): 289-300.
- Ferreira FV, Cividanes LS, Gouveia RF, Lona LMF (2019) An overview on properties and applications of poly (butylene adipate- co -terephthalate)-PBAT based composites, Polym Eng Sci 59(s2): E7-E15.
- Muthuraj R, Misra M, Mohanty AK (2018) Biodegradable compatibilized polymer blends for packaging applications: A literature review. J Appl Polym Sci 135(24).
- Zhong Y, Godwin P, Jin Y, Xiao H (2020) Biodegradable polymers and green-based antimicrobial packaging materials: A mini-review. Advanced Industrial and Engineering Polymer Research 3(1): 27-35.
- Rusmirović JD, Kovačević TM, Brzić SJ, Marinković AD (2020) Cross-linkable bio and mineral fillers for reactive polymer composites: processing and characterization. Reactive and Functional Polymers Volume Two, pp. 135-163.
- Zhang HZ, He ZC, Liu GH, Qiao YZ (2009) Properties of different chitosan/low-density polyethylene antibacterial plastics. J Appl Polym Sci 113(3): 2018-2021.
- Cazón P, Velazquez G, Ramírez JA, Vázquez M (2017) Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocolloids 68: 136-148.
- Wang H, Qian J, Ding F (2018) Emerging chitosan-based films for food packaging applications. J Agric Food Chem 66(2): 395-413.
- Dutta PK, Tripathi S, Mehrotra GK, Dutta J (2009) Perspectives for chitosan based antimicrobial films in food applications. Food Chemistry 114(4): 1173-1182.
- van den Broek LAM, Knoop RJI, Kappen FHJ, Boeriu CG (2015) Chitosan films and blends for packaging material. Carbohydrate Polymers 116: 237-242.
- Ravi Kumar MNV (2000) A review of chitin and chitosan applications. Reactive and Functional Polymers 46(1): 1-27.
- Shukla SK, Mishra AK, Arotiba OA, Mamba BB (2013) Chitosan-based nanomaterials: A state-of-the-art review. International Journal of Biological Macromolecules 59: 46-58.
- Divya K, Jisha MS (2018) Chitosan nanoparticles preparation and applications. Environ Chem Lett 16: 101-112.
- Matet M (2014) PhD Thesis, Ecole Polytechnique de Montré
- Correlo VM, Boesel LF, Bhattacharya M, Mano JF, Neves NM, et al. (2005) Properties of melt processed chitosan and aliphatic polyester blends. Materials Science and Engineering 403(1-2): 57-68.
- Bonilla J, Fortunati E, Vargas M, Chiralt A, Kenny JM (2013) Effects of chitosan on the physicochemical and antimicrobial properties of PLA films. Journal of Food Engineering 119(2): 236-243.
- Díez-Pascual AM, Díez-Vicente AL (2015) Antimicrobial and sustainable food packaging based on poly (butylene adipate-co-terephthalate) and electrospun chitosan nanofibers. RSC Adv 5: 93095-93107.
- Bie P, Liu P, Yu L, Li X, Chen L, et al. (2013) The properties of antimicrobial films derived from poly(lactic acid)/starch/chitosan blended matrix. Carbohydrate Polymers 98(1): 959-966.
- Cesur S, Köroğlun C, Yalçın HT (2018) Antimicrobial and biodegradable food packaging applications of polycaprolactone/organo nanoclay/chitosan polymeric composite films. J Vinyl Addit Technol 24(4): 376-387.
- Râpă M, Miteluţ AC, Tănase EE, Grosu E, Popescu P, et al. (2016) Influence of chitosan on mechanical, thermal, barrier and antimicrobial properties of PLA-biocomposites for food packaging. Composites Part B: Engineering 102: 112-121.
- Ghanbari A, Tabarsa T, Ashori A, Shakeri A, Mashkour M (2018) Preparation and characterization of thermoplastic starch and cellulose nanofibers as green nanocomposites: Extrusion processing, International Journal of Biological Macromolecules 112: 442-447.
- Matet M, Heuzey MC, Pollet E, Ajji A, Avérous L (2013) Innovative thermoplastic chitosan obtained by thermo-mechanical mixing with polyol plasticizers. Carbohydrate Polymers 95(1): 241-251.
- Meng Q, Heuzey MC, Carreau PJ (2014) Hierarchical structure and physicochemical properties of plasticized chitosan. Biomacromolecules 15(4): 1216-1224.
- Zhang Y, Liu BL, Wang LJ, Deng YH, Zhou SY, et al. (2019) Preparation, structure and properties of acid aqueous solution plasticized thermoplastic chitosan. Polymers.
- Epure V, Griffon M, Pollet E, Avérous L (2011) Structure and properties of glycerol-plasticized chitosan obtained by mechanical kneading. Carbohydrate Polymers 83(2): 947-952.
- Sun K, Li F, Li J, Li J, Zhang C, et al. (2019) Optimisation of compatibility for improving elongation at break of chitosan/starch films. RSC Adv 42(9): 24451-24459.
- Dang KM, Yoksan R (2015) Development of thermoplastic starch blown film by incorporating plasticized chitosan. Carbohydrate Polymers 115: 575-581.
- Mendes JF, Paschoalin RT, Carmona VB, Sena Neto AR, Marques ACP, et al. (2016) Biodegradable polymer blends based on corn starch and thermoplastic chitosan processed by extrusion. Carbohydrate Polymers 137: 452-458.
- Matet M, Heuzey MC, Ajji A (2014) Morphology and antibacterial properties of plasticized chitosan/metallocene polyethylene blends. J Mater Sci 49: 5427-5440.
© 2022 H de Baynast. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






