- Submissions

Full Text
Polymer Science: Peer Review Journal
Fabrication of Rechargeable Lithium Ion Coin Cell using Triblock Polymer Electrolyte Poly (Vinylidene Chloride-Co-Acrylonitrile- Co-Methyl Methacrylate) with Lithium Perchlorate
Vengadesh Krishna M1,2, Chitra R2,3 and Selvasekarapandian S2,4*
1Department of Chemistry, Government Arts College Coimbatore-641018, Tamil Nadu, India
2Materials Research Center, Coimbatore, Tamilnadu, India
3Department of Physics, Kongu Arts and Science College (Autonomous), Erode, Tamil Nadu, India
4Department of Physics, Bharathiar University, Coimbatore, Tamilnadu, India
*Corresponding author: Selvasekarapandian S, Department of Physics, Bharathiar University, Coimbatore, Tamilnadu,India
Submission: September 26, 2020;Published: November 17, 2020

ISSN: 2770-6613 Volume1 Issue2
Abstract
Lithium ion conducting polymer electrolytes based on triblock polymer poly(vinylidene chloride-co-acrylonitrile- co-methyl methacrylate) (or) P(VdCl-co-AN-co-MMA)-LiClO4 with tetrahydrofuran (THF) as solvent have been prepared by solution casting technique. Electrochemical impedance analysis of the polymer membranes reveals that the ionic conductivity of the polymer electrolyte increases with increase of LiClO4salt composition and the highest ionic conductivity of 1.9x10-3Scm-1 is observed for 40 wt% P(VdCl-co-AN-co-MMA)/60 wt% LiClO4 at room temperature. Alithium ion coin cell has been fabricated with a configuration of Graphite||40m%P(VdCl-co-AN-co-MMA)/60 m% LiClO4||LiFePO4and a maximum cell potential of 3.15V is observed which affirms applicability of membrane for solid state lithium ion batteries.
Keywords: Polymer; Lithium; Electrolytes; Discharging
Introduction
Battery electrolytes shuttle ions between the electrodes during charging and discharging process. Especially in lithium ion batteries, search of electrolyte remains challenging aspect. Solid electrolytes in the place of liquid electrolytes hold promise for safety, long lasting and shape and design flexibility [1,2]. In this perspective, lithium ion conducting polymer electrolytes based on triblock polymer poly (vinylidene chloride-co-acrylonitrile-co-methyl methacrylate) (or) P(VdCl-co-AN-co-MMA) have been focused. The choice of host polymer P(VdCl-co-AN-co-MMA), here after mentioned as P(VdCl..) is owing to the presence of huge number of polar groups such as oxygen, nitrogen and chlorine [3]. It is a known fact that salt as ion provider significantly take part in determining the characteristic of polymer electrolytes such as conductivity, thermal and mechanical stability. In the present work, lithium perchlorate (LiClO4) salt has been selected as ionic dopant due to this low molecular weight and low dissociation energy [4]. The present investigation aims to fabricate lithium ion full cell (CR 2032 coin cell) with highest conducting polymer membrane and explore the performance of the rechargeable lithium ion cell.
Experimental Method
Triblock copolymer P(VdCl.), LiClO4 salt and tetrahydrofuran (THF) were purchased from Sigma-Aldrich. Appropriate amount of P(VdCl.) and LiClO4 is dissolved in THF solvent at room temperature and stirred continuously. A homogeneous viscous liquid is obtained after continuous stirring for 4 days. The homogeneous liquid is transferred into petri dish and left undisturbed for evaporation. Finally, solid polymer electrolytes of composition 60 wt% P(VdCl.): 40 wt% LiClO4, 50 wt% P(VdCl.): 50 wt% LiClO4, 40 wt% P(VdCl.): 60 wt% LiClO4 and 30 wt% P(VdCl.): 70 wt % LiClO4 have been prepared by solution casting method.
Characterization
Prepared solid polymer electrolyte samples have been characterized by Electrochemical Impedance (EIS) analysis using HIOKI 3532-50 LCR HITESTER. The electrolyte samples have been sandwiched between two stainless-steel blocking electrodes over the frequency range of 42Hz to 5MHz.
Fabrication of CR2032 lithium ion coin cell
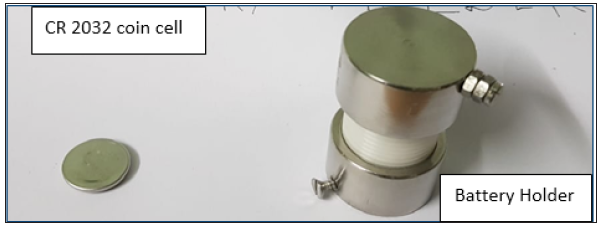
Cathode slurry has been prepared with a mixture of active material of lithium iron phosphate, acetylene black and PVDF binder in the weight ratio of 8:1:1. N-methylpyrrolidone (NMP) has been used as a solvent. Cathode slurry has been coated over aluminium foil and cut into the disk of required size. Battery grade graphite powder has been coated over copper foil to fabricate anode electrode. A coin cell has been fabricated with a configuration of Graphite||40 wt% P(VdCl.): 60 wt% LiClO4||LiFePO4. The charge-discharge cycles of fabricated coin cell has been analyzed by placing it in a battery holder. The photograph of the fabricated coin cell and the battery holder is shown in Figure 1.
Figure 1: Photograph of the fabricated coin cell and the battery holder.
Results and Discussion
EIS analysis
The ionic conductivity of the electrolytes has been determined using AC impedance technique at room temperature. A plot between the real and imaginary part of impedance as a function of frequency has been obtained and is complex impedance plot or Nyquist plot or cole-cole plot. Ionic conductivity studies for P(VdCl.) with MgCl2 has been reported by Ponraj et al and the ionic conductivity value for pure P(VdCl.) is 3.07×10−8Scm-1 [3]. In the present investigation, ionic conductivity values of prepared polymer electrolytes have been obtained using
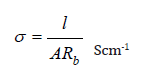
Figure 2 shows the Nyquist plots of various compositions of polymer electrolytes. It is obvious that diminishing of semicircle is established with the increasing salt content. Almost, a spike is observed for the polymer composition of 40 wt% P(VdCl.): 60 wt% LiClO4 and the high ionic conductivity of 1.97x10-3Scm-1 is exhibited by this sample. (Table 1) provides the bulk resistance and ionic conductivity values of prepared electrolytes.
Figure 2: Nyquist plots of various compositions of polymer electrolytes.
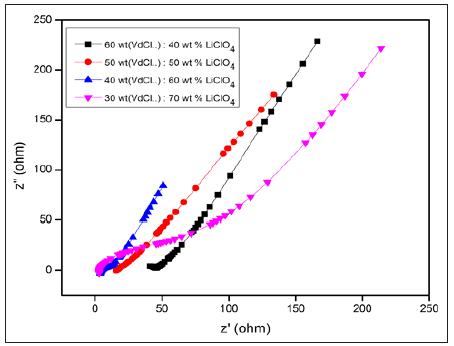
Table 1: Bulk Resistance & Ionic conductivity values of polymer electrolytes.

Charge-discharge characteristic of full cell
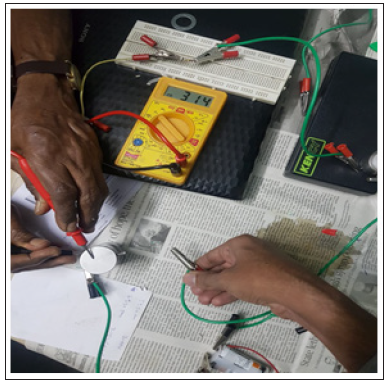
The fabricated cell is subjected to DC supply of 4V. Open circuit cell potential of 3.15V has been observed for the first cycle and then the cell has been charged for one hour. The discharge of the cell takes 4 hours to attain 0.6V. At the 15th cycle, the cell has been charged to the maximum of 1.75V and the same discharge time is observed for this cycle too. The value of the cell resistance has been found to increase with charging cycles owing to interface resistance between electrodes and electrolyte of the cell. The Nyquist plot of fabricated coin cell at 10th and 15th cycle is shown in Figure 3 and the photograph of the fabricated cell potential is shown in Figure 4.
Figure 3: Nyquist plot of fabricated coin cell.

Figure 4: Photograph of the fabricated cell potential.
Conclusion
The flexible solid polymer electrolytes of P(VdCl.): LiClO4 have been prepared by the solution casting technique and the better ionic conductivity of the order of 10-3Scm-1 is obtained by the composition 40 wt% P(VdCl.): 60 wt% LiClO4. A fabricated full cell displayed a good charging –discharging cycles and proves the applicability of the membrane in lithium ion battery.
References
- Pandey M, Joshi GM, Ghosh NN (2016) Ionic conductivity and diffusion coefficient of barium-chloride-based polymer electrolyte with poly(vinyl alcohol)-poly(4-styrenesulphonic acid) polymer complex. Bull Mater Sci 40(4): 655-666.
- Meyer WH (1998) Polymer electrolytes for lithium-ion batteries. Adv Mater 10(6): 439-448.
- Ponraj T, Ramalingam A, Selvasekarapandian S, Srikumar SR, Manjuladevi R (2019) Plasticized solid polymer electrolyte based on triblock copolymer poly(vinylidene chloride co acrylonitrile co methyl methacrylate) for magnesium ion batteries. Polymer Bulletin.
- Perumal P, Selvasekarapandian S, Abhilash KP, Sivaraj P, Hemalatha R, et al. (2018) Impact of lithium chlorate salts on structural and electrical properties of natural polymer electrolytes for all solid state lithium polymer batteries. Vacuum 159: 277-281.
© 2020 Selvasekarapandian S. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






