- Submissions

Full Text
Peer Review Journal of Solar & Photoenergy Systems
Advances in Solar-Photocatalytic Removal of Pollutants From Pharmaceutical Industrial Wastewater
Muzammil Anjum1*, Fareena Batool1, Mobeena Anees1, Shaffiullah1, Rab Nawaz1 and Samia Qadeer2
1Institute of Soil and Environmental Sciences, PMAS Arid Agriculture University, Pakistan
2Department of Environmental Sciences, Allama Iqbal Open University, Pakistan
*Corresponding author: Muzammil Anjum, Institute of Soil and Environmental Sciences, PMAS Arid Agriculture University, Pakistan
Submission: March 17, 2023;Published: April 27, 2023

Volume2 Issue3 April , 2023
Abstract
The countless existence of pharmaceutical pollutants discharged wastewater has been identified as a potential concern for aquatic creatures and humans. Despite the fact that their presence in drinking water has caused significant concern, little is known about their fate and environmental impact. As a result, these contaminants are inexorably pushed into our food chain even at low levels. Pharmaceutical effluents have a both direct and indirect impact on environmental and human health, especially in the vicinity of pharma industrial zones. Therefore, it is important to remove pharmaceutical pollutants from wastewater before final discharge in the environment. Among various treatment, photocatalysis has been widely regarded and gained popularity in recent years as a potential technology for removal of variety of environmental contaminants. The present mini review mainly focused on the feasibility of photocatalysis process for its potential to treat pharmaceutical wastewater. Moreover, the emphasis is given to the solar based (visible light active) photocatalyst such as C3N4 and its composites.
Keywords: Pharmaceutical pollutants; Photocatalysis; Nano-Composites; C3N4; Wastewater
Introduction
Pharmaceutical pollutants have a direct impact on human health because they have been discovered in our food chain, which includes fruits, vegetables, and drinking water [1,2]. The pharmaceutical department has emerged as one of the key benefactors of this trend, as the use of pharmaceuticals and public-health products has been made more affordable and accessible. Pharmaceuticals medicines play very important character for people life insurance and life quality because they are used in curing various health concerns for instance; pregnancy prevention, stress, both mental and physical, fever, infection and agricultural growth every year [3,4]. Every year, the rate of use of pharmaceutical drugs has been continuously increasing globally by 11.9 % such as in USA, France, Japan, United Kingdom, Spain, and Italy etc. [2,5]. Due to this, pharmaceutical contaminants are found in aquatic habitats and animal tissues [6]. The vital downside connected with such a vast utilization of pharmaceutical objects is their unlimited excretion into both marine and terrestrial ecosystem [1]. One of the most common contaminants is fluoxetine antidepressants, which may affect behavioral and physiological processes in non-target individuals [6]. Moreover, a considerable concentration of endocrine disrupting chemicals is also classified among the pollutants that are on the rise [1].
Pharmaceutical chemicals typically enter natural water systems through the overflow of different non-point water sources, such as agricultural activities or static water supplies, such as municipal and hospital wastewater treatment plants [7] (Okoye et al., 2022). In human drinking water there are possibility of presence of pharmaceutical compounds consists of various sources such as pharmaceutical production business, process of production, use and application by human and veterinary. In the above context, it is important to remove the pharmaceutical compounds from water in order to protect its secondary impact on human and environmental health. Several conventional wastewater cure methods for detach pharmaceutical compounds have been notified like activated sludge, adsorption, ozonation, wetlands, and microalgae [1]. Biological treatment methods are wieldy applied for the treatment of industrial wastewater. The utilization of activated sludge and trickling filters for the disposal of pharmaceutical wastewater proved ineffective, resulting in the wastewater being released into the environment and constantly polluting surface, soil, and groundwater [7]. Furthermore, traditional biological wastewater treatment systems remain in short supply for entirely separating refractory toxins from pharmaceutical wastewater (Inyang et al. 2016). Although, the primary treatment techniques for pharmaceutical wastewater treatment are physio-chemical and biological methods, however, the biological treatment is less expensive, but it is less efficient in carbon-based pollutants in wastewater.
Photocatalysis has gained popularity in recent years as a potential technology for resolving vitality issues and preventing environmental pollution [8-13]. Photo-catalysis is a form of chemical reaction that is extremely advantageous due to its ease of use, inexpensive safety, effective degradation, and perfect stability. The primary application areas in catalysis, particularly in wastewater treatment, include photocatalytic electrolysis, environmental protection, solar cells, storage apparatus, and others. In case of wastewater treatment, TiO2 photo catalysis has been proved to efficiently degrade the organic pollutants [2,14]. The catalytic activity of TiO2 is based on its photoelectric characteristics and electrical structure. The photo-catalytic response principle is defined using the band theory in which the catalyst is activated under the continuous illumination of light with the energy equal to or higher than the band gap energy of catalyst, resulting in generation of a hole-electronic pair for oxidation and reduction of pollutants.
Environmental Impacts of Pharmaceutical Pollutants
Pharmaceutical substances are utilized for a variety of beneficial uses in industrial life, but they often release highly toxic chemicals into the atmosphere, either directly or as a result of chemical changes [15]. Pharmaceutical medications, both for medical and veterinary medicine, are posing a threat to the environment. Analgesics, antibiotics, antiepileptics, antiseptics, beta-blockers, antihypertensive, hormones, contraceptives, psycho therapeutics, and antivirals are some of the pharmaceuticals that have been listed. Any medications in the atmosphere even at low concentrations will damage aquatic species [16]. Diclofenac was then extensively researched in other bird species (pigeons and hens), with death rates of 0.25mg kg1 in pigeons and 2.5mg kg1 in chickens [17]. It’s thought that diclofenac’s toxicity causes an increase in reactive oxygen species formation and, as a result, a shift in uric acid metabolism (creation and elimination) [18] Fish acute toxicity values are expected to reach 100mg l 1. During a 28-day test, however, it was revealed that at a dose of 1mg l 1, harmful effects on rainbow trout may already have begun. River trout have been shown to be harmed by 50mg/1 dosages (Hoeger et al. 2005). The conversion of these medications to compounds with features similar to their maternal molecules and the ability to bio-accumulate in the tissues is also a concern for the environment [19]. Under laboratory conditions, carbamazepine has an effect on a zebra mussel (Dreissena polymorpha) [20]. They discovered that diclofenac in the vultures’ food web contributed to their extinction [21]. Pharmaceuticals enter the atmosphere through processing units and patient effluents, as well as applications for land (e.g., biosolids and water reuse).
Sewage treatment services, on the other hand, aren’t always effective in separating active chemicals from waste water. As a result, pharmaceuticals end up in the marine ecosystem, where they have direct effects on aquatic life and can enter food chains. Several drugs were detected in extremely high amounts (mg/L) in effluents from a local wastewater treatment plant near Visakhapatnam, India, in a recent report [15]. Trace level concentrations have also been found in microorganisms, fish, mollusks, and rodents, but in fewer publications. The pharmaceutical industry’s waste water has a strong tint, a pungent taste, a low BOD and a high COD.
The various wastewater channels from the active pharmaceutical sector, unpackaged drugs, and associated pharmaceuticals, all of which use a lot of water, must be recognized, and the best technologies for eliminating them must be analyzed in order to increase public quality and water supplies [7]. Researchers have identified high quantities of pharmaceutical chemicals in a variety of wastewater types, including ground and surface waters, as well as drinking water sources (Yang et al.,2014). As a result, the environmental influence and public health threats associated with these forms of wastewater are being studied by experts all over the world.
Pharmaceutical compounds typically enter natural water supplies through source of non-point water such as agronomic activity or source of constant water such as wastewater treatment plants in municipalities and hospitals [7] (Okoye et al., 2022). Massive concentrations of heavy metal ions, radionuclides, toxins, products of personal care, and chronic chemical chemicals are eventually released into the natural world as a result of the rapid growth of industry, urban growth, and cultivation.
Photocatalysis Process
Photo-catalysis has been widely regarded in recent years as a potential technology for resolving vitality issues and preventing environmental pollution. Photo-catalytic electrolysis of water, environmental protection, solar cells, storehouse apparatus, and so on are the main application areas in catalysis, especially in wastewater treatment. Organic pollutants such as pesticides, dyes, and chemical waste can be found of large quantities in wastewater, causing severe damage to biological health and ecological practices. Organic contaminants that are particularly toxic and slow to degrade (such as contaminants, fertilizer, and dyes) have a long halflife, and even their exploration may cause biological differences. As a green catalytic mechanism, TiO2 photo-catalysis will almost fully degrade all organic pollutants. TiO2 photo-catalytic methods can degenerate more than 3000 forms of problems decomposing organic composites, according to research [22].
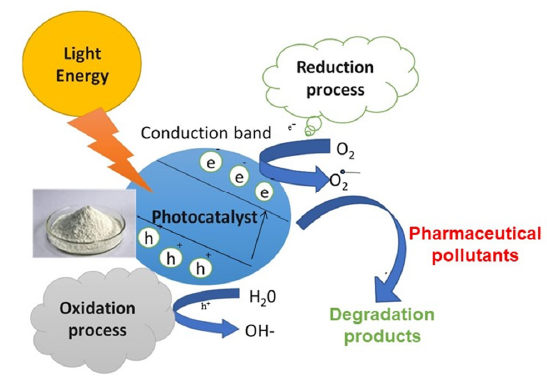
The examination of visible-light active semiconductor photocatalysts has obtained great use in environmental nanotechnology. Several conventional wastewater cure methods for detach PhACs and EDCs have been notified such as activated sludge, adsorption, wetlands, ozonation, and micro-algae [1]. Photo-catalysis is a form of chemical reaction that is extremely advantageous due to its ease of use, low cost, safety, high degradation efficiency and perfect stability. TiO2’s catalytic activity is based on its electronic structure and photoelectric properties. The photo-catalytic response principle can be defined using the band theory. The photo-catalytic response principle can be defined using the band theory. When exterior TiO2 is illuminated with light that is consistently equal to or greater than the band gap energy of TiO2, the surface is stimulated, and a hole-electronic pair is formed, which allows for oxidation and reduction. The general mechanism of the photocatalysis process is shown in Figure 1.
Figure 1:Photocatalysis general mechanism.
C3N4 Based Photocatalysts
Since its ground breaking use in photo-catalysis in 2009, graphitic phase carbon nitride (g-C3N4) has become a research hotspot as a novel kind of nonmetal and stable polymeric semiconductor content [23,24]. Exfoliation of the bulk structure of g-C3N4 is one of the techniques for improving its photo-catalytic performance. Exfoliation increases the basic surface area of these g-C3N4 structures, which improves their photo-catalytic activity greatly [25]. The use of g-C3N4 for photo-catalytic deterioration of the anti-inflammatory pharmaceuticals (DIC, PAR, and IBU) is still being studied. Diclofenac degradation is reported in just a few articles [26]. The g-C3N4 as a traditional synthetic material has become a hot topic in the field of chemistry and material science, particularly for photo-catalysis, due to its low cost, ease of preparation, strong stability, and unusual physicochemical properties [27,28]. The g-C3N4 materials are successfully formed by air flow rate increases with small organic molecules that are nitrogen-rich precursors, such as urea, cyanimide, melamine, dicyanamide and thiourea, and the form of tectonic units depends primarily on the reaction processes [29]. The most successful method for purifying wastewater contaminated by organic contaminants is the adsorption and photo-catalytic oxidation of organic molecules under visible light irradiation. The structure properties and adsorption capacities, in general, dominate photo-catalytic capacity. As a result, the majority of the literature on the elimination of organic compounds focused on adsorption and photo-catalytic degradation. The photocatalytic performance of various C3N4 based photocatalysts is summarized in Table 1; [30-37].
Table 1:Photocatalytic removal of various pollutants by modified C3N4 photocatalysts.

Modification of Catalyst for Visible Light (Solar) Photocatalysis
Since it can induce the formation of hydroxyl radicals from H2O2, graphitic carbon nitride (g-C3N4) is regarded as a Fentonlike catalyst. g-C3N4 is also a widely used photo-catalyst for water splitting and organic degradation (Li et al., 2016; Dong et al., 2017). However, the poor separation performance of charge carriers as photo-catalysts and the low utilization efficiency of H2O2 as a Fenton-like catalyst limit g-use C3N4’s [38,39]. While alkalinization and metal ions alteration will improve the Fenton-like catalytic and photo-catalytic capability of g-C3N4, the redox potential is still limited from a thermodynamic perspective. The use of Z-scheme hetero-junctions can increase the spectrum of light absorption, facilitate charge carrier separation, and improve photo-catalyst redox efficiency. Numerous Z-scheme hetero-junctions based on g-C3N4, such as g-C3N4@C-TiO2 and g-C3N4/Ag3PO4/AgI, have been developed [40,41]. Replication of holes (h+) in the Valence Band (VB) of g-C3N4 with Electrons (e-) in the Conduction Band (CB) of another semiconductor gives the e- in the CB of g-C3N4 and the h+ in the VB of another semiconductor super removing and oxidizing capabilities, simultaneously. Using a calcination - impregnation process, we formed a novel Z-scheme MnO2/Mn-modified alkalinized g-C3N4 catalyst (MnO2/CNK-OH-Mn). MnO2/CNK-OHMn displayed strong Fenton-like photo-catalytic activity, with high Tetracycline (TC) degradation and TOC removal performance, as well as COD extraction efficiency in pharmaceutical wastewater [42].
TiO2 modified C3N4
The photocatalytic degradation of paracetamol, ibuprofen, and diclofenac was investigated using TiO2 nanoparticles as well as exfoliated g-C3N4. Their aqueous solutions were first exposed for 2 hours to UV and VIS radiation. When the adsorption–desorption equilibrium between medicines and nanomaterials was achieved, the photocatalytic studies began (after dark). The medicines did not undergo photolysis [43]. Because of low toxicity, high durability, low price, and environmental advantages, photo-catalysis has gotten a lot of publicity. As previously said, titanium dioxide (TiO2) is a prototype photo-catalyst that has been used to decompose a wide range of pollutants [44,45]. However, because of their ease of agglomeration, limited UV activity, and high recombination rate of photo-generated e-h+ pairs, pristine TiO2 nanoparticles have some limitations in practical use [46,47] (Li et al., 2018a). As a result, the semiconductor/TiO2 construction may be considered to increase photo-catalytic activity.
This photo-catalyst is powered by visible light and has a suitable band gap (2.70eV) [48]. This carbon-doped supramolecule-based g-C3N4 (BCCN) and TiO2 hybrids can therefore be used to improve visible-light-driven photo-degradation pharmaceuticals. LED lamps, on the other hand, seem to be promising sites of pollutant degradation [49,50]. An in-situ process was used to fabricate an ecofriendly 2D hetero-junction photo-catalyst composites (BCCNT) made up of carbon-doped supra-molecule-based g-C3N4 (BCCN) layers and TiO2 nano particles (Hu et al., 2019). The photo-catalytic degradation of paracetamol (PAR), ibuprofen (IBU), and diclofenac was studied using exfoliated graphitic carbon nitride (g-C3N4) and two commercially usable titanium dioxide nano materials (P25 and CG300) (DIC) [43].
Due to the breakdown of intermediate intermediates, a slightly pinkish solution was noticed after 2–3h during diclofenac photodegradation, which disappeared with a longer irradiation period. There was no discernible effect in the case of paracetamol. In the sequence g-C3N4 CG300 P25, photocatalyst degradation effciency for all medicines increased. When considering the wavelength of 368nm and the band gap energies of TiO2 nanoparticles, this was not surprising. Because of its rapid photoinduced electron-hole recombination, g-C3N4 has the lowest photodegradation activity. The degradation effciency of g-C3N4 was around 77 percent (VIS) and 7% (UV), which corresponded to a band gap energy of 2.70eV. (459nm). As a result, g-C3N4 should be active in both UV (368nm) and VIS (446nm). Because none of these compounds absorbed visible light at 446nm, the photodegradation in the presence of TiO2 could not be attributable to sensitization by the adsorbed medicines, as was commonly reported in the case of different dyes. The VIS light absorption of both TiO2 nanomaterials, as a result of structural flaws such as oxygen vacancies, most likely the cause. The oxygen vacancies are inherent defects that produce intermediate energy levels inside the TiO2 band gap and function as a recombination site for photo induced electron and hole recombination. The absorption spectra of TiO2 suspensions, both pure TiO2 nanomaterial and their mixtures, with the medication are displayed [43]. In order to get the right quantifiable absorbance, the P25 solution was diluted 60 times and the CG300 suspension was diluted 15. Before the photocatalytic process began, the spectra were collected after 1 hour of darkness. TiO2 absorbed visible light in both cases, most likely due to electron transfer from their valence band to the intermediate energy level of oxygen vacancies.
Conclusion
In the current environment, modern water technologies are required to maintain high water quality, remove chemical and biological contaminants, and accelerate industrial waste production processes. Photocatalysis is one of the best alternatives for advanced wastewater treatment in this regard. For wastewater treatment, several nano-materials have been effectively produced and researched. Under the influence of visible light, a novel visible light photocatalyst C3N4 effectively synthesized, with the capacity to degrade antibiotics and remove pathogens from pharmaceutical industrial wastewater. Nano-particle photo-catalysts can be utilized to treat both hazardous contaminants and heavy metals, with the capacity to employ visible sunlight instead of expensive artificial UV radiation due to changes in catalyst material. The catalyst C3N4 demonstrated excellent photo-catalytic activity as well as good stability, thus can be used for effective treatment of wastewater.
References
- Chauhan A, Sillu D, Agnihotri S (2019) Removal of pharmaceutical contaminants in wastewater using nanomaterials: A comprehensive review. Current drug metabolism 20(6): 483-505.
- Eslami A, Amini MM, Asadi A, Safari AA, Daglioglu N (2020) Photocatalytic degradation of ibuprofen and naproxen in water over NS-TiO2 coating on polycarbonate: Process modeling and intermediates identification. Inorganic Chemistry Communications 115: 107888.
- Younas A, Naqvi SA, Khan MR, Shabbir MA, Jatoi MA, et al. (2020) Functional food and nutra‐pharmaceutical perspectives of date (Phoenix dactylifera L.) Journal of Food Biochemistry 44(9): e13332.
- González RB, Sharma P, Singh SP, Américo P, Parra SR, et al. (2022) Persistence, environmental hazards, and mitigation of pharmaceutically active residual contaminants from water matrices. Science of The Total Environment 821: 153329.
- Kaur A, Umar A, Kansal SK (2016) Heterogeneous photocatalytic studies of analgesic and non-steroidal anti-inflammatory drugs. Applied Catalysis A: General 510: 134-155.
- Martin JM, Bertram MG, Saaristo M, Ecker TE, Hannington SL, et al. (2019) Impact of the widespread pharmaceutical pollutant fluoxetine on behaviour and sperm traits in a freshwater fish. Science of the Total Environment 650(Pt 2): 1771-1778.
- Zaied BK, Rashid M, Nasrullah M, Zularisam AW, Pant D, et al. (2020) A comprehensive review on contaminants removal from pharmaceutical wastewater by electrocoagulation process. Science of the Total Environment 726: 138095.
- Barakat MA, Anjum M, Kumar R, Alafif ZO, Oves M, et al. (2020) Design of ternary Ni(OH)2/graphene oxide/TiO2 nanocomposite for enhanced photocatalytic degradation of organic, microbial contaminants, and aerobic digestion of dairy wastewater. Journal of Cleaner Production 258: 120588.
- Alafif ZO, Anjum M, Kumar R, Abdelbasir SM, Barakat MA (2019) Synthesis of CuO-GO/TiO2 visible light photocatalyst for 2-chlorophenol degradation, pretreatment of dairy wastewater and aerobic digestion. Applied Nanoscience 9: 579-591.
- Anjum M, Kumar R, Abdelbasir SM, Barakat MA (2018) Carbon nitride/titania nanotubes composite for photocatalytic degradation of organics in water and sludge: Pre-treatment of sludge, anaerobic digestion and biogas production. Journal of Environmental Management 223: 495-502.
- Anjum M, Kumar R, Al-Talhi HA, Mohamed SA, Barakat MA (2018) Valorization of biogas production through disintegration of waste activated sludge using visible light Zno-Zns/Ag2o-Ag2s photocatalyst. Process Safety and Environmental Protection 119(1): 330-339.
- Anjum M, Miandad R, Waqas M, Gehany F, Barakat MA (2019) Remediation of wastewater using various nano-materials. Arabian Journal of Chemistry 12(8): 4897-4919.
- Anjum M, Liu W, Qadeer S, Khalid A (2023) Photocatalytic treatment of wastewater using nanoporous aerogels: Opportunities and challenges. Emerging Techniques for Treatment of Toxic Metals from Wastewater 495-523.
- Lopez IP, Pugliese D, Boetti NG, Janner D, Baldi G, et al. (2018) Design, synthesis, and structure-property relationships of Er3+-doped TiO2 luminescent particles synthesized by sol-gel. Nanomaterials 8(1): 20.
- Patneedi CB, Prasadu, KD (2015) Impact of pharmaceutical wastes on human life and environment. Rasayan Journal of Chemistry 8(1): 67-70.
- Petrovic M, Perez S, Barcelo D (2013) Analysis, removal, effects and risk of pharmaceuticals in the water cycle: Occurrence and transformation in the environment. (2nd edn), Elsevier, Amsterdam, Netherlands.
- Hussain I, Khan MZ, Khan A, Javed I, Saleemi MK (2008) Toxicological effects of diclofenac in four avian species. Avian Pathology 37(3): 315-321.
- Paul-Murphy J, Ludders JW (2001) Avian analgesics. Veterinary Clinics of North America: Exotic Animal Practice 4(1): 35-45.
- Mackuak T, Černanský S, Fehér M, Birošová L, Gál M (2019) Pharmaceuticals, drugs, and resistant microorganisms-environmental impact on population health. Current Opinion in Environmental Science & Health 9: 40-48.
- Contardo-Jara V, Lorenz C, Pflugmacher S, Nützmann G, Kloas W, et al. (2011) Exposure to human pharmaceuticals Carbamazepine, Ibuprofen and Bezafibrate causes molecular effects in Dreissena polymorpha. Aquatic Toxicology 105(3-4): 428-437.
- Oaks JL, Gilbert M, Virani MZ, Watson RT, Meteyer CU, et al. (2004) Diclofenac residues as the cause of vulture population decline in Pakistan. Nature 427(6975): 630-633.
- Jiang L, Wang Y, Feng C (2012) Application of photocatalytic technology in environmental safety. Procedia Engineering 45: 993-997.
- Cao S, Low J, Yu J, Jaroniec M (2015) Polymeric photocatalysts based on graphitic carbon nitride. Advanced Materials 27(13): 2150-2176.
- Yan SC, Li ZS, Zou ZG (2009) Photodegradation performance of g-C3N4 fabricated by directly heating melamine. Langmuir 25: 10397-10401.
- Svoboda L, Praus P, Lima MJ, Sampaio MJ, Matýsek D, et al. (2018) Graphitic carbon nitride nanosheets as highly efficient photo-catalysts for phenol degradation underhigh-power visible LED irradiation. Mater Res Bull 100: 322-332.
- Moreira NFF, Sampaio MJ, Ribeiro AR, Silva CG, Faria JL, et al. (2019) Metal-free g-C3N4 photocatalysis of organic micropollutants in urban wastewater under visible light. Appl Catal B: Environ 248.
- Patnaik S, Sahoo DP, Parida K (2018) An overview on Ag modified g-C3N4 based nanostructured materials for energy and environmental applications. Renewable and Sustainable Energy Reviews 82(Pt 1): 1297-1312.
- Hayat A, Sohail M, Anwar U, Taha TA, Qazi HIA, et al. (2023) A targeted review of current progress, challenges and future perspective of g‐C3N4 based hybrid photocatalyst toward multidimensional applications. The Chemical Record 23(1): e202200143.
- Hayat A, Sohail M, Ali Shah Syed J, Al‐Sehemi AG, Mohammed MH, et al. (2022) Recent advancement of the current aspects of g‐C3N4 for its photocatalytic applications in sustainable energy system. The Chemical Record 22(7): e202100310.
- Sun H, Zou C, Tang W (2022) Designing double Z-scheme heterojunction of g-C3N4/Bi2MoO6/Bi2WO6 for efficient visible-light photocatalysis of organic pollutants. Colloids and Surfaces A: Physicochemical and Engineering Aspects 654: 130105.
- Liyanaarachchi H, Thambiliyagodage C, Liyanaarachchi C, Samarakoon U (2023) Efficient photocatalysis of Cu doped TiO2/g-C3N4 for the photodegradation of methylene blue. Arabian Journal of Chemistry 104749.
- Kong H, Li H, Wang H, Li S, Lu B, et al. (2023) Fe-Mo-O doping g-C3N4 exfoliated composite for removal of rhodamine B by advanced oxidation and photocatalysis. Applied Surface Science 610: 155544.
- Qing Y, Li Y, Guo Z, Yang Y, Li W (2022) Photocatalytic Bi2WO6/pg-C3N4-embedded in polyamide microfiltration membrane with enhanced performance in synergistic adsorption-photocatalysis of 17β-estradiol from water. Journal of Environmental Chemical Engineering 10(6): 108648.
- Berhanu S, Gebremariam H, Chufamo S (2022) The g-C3N4@ CdO/ZnO ternary composite:0020Photocatalysis, thermodynamics and acute toxicity studies. Heliyon 8(11): e11612.
- Jing M, Zhao H, Jian L, Pan C, Dong Y, et al. (2023) Coral-like B-doped g-C3N4 with enhanced molecular dipole to boost photocatalysis-self-Fenton removal of persistent organic pollutants. Journal of Hazardous Materials 449: 131017.
- Ma Y, Li J, Cai J, Zhong L, Lang Y, et al. (2022) Z-scheme g-C3N4/ZnS heterojunction photocatalyst: One-pot synthesis, interfacial structure regulation, and improved photocatalysis activity for bisphenol A. Colloids and Surfaces A: Physicochemical and Engineering Aspects 653: 130027.
- Yu Y, Hu X, Li M, Fang J, Leng C, et al. (2022) Constructing mesoporous Zr-doped SiO2 onto efficient Z-scheme TiO2/g-C3N4 heterojunction for antibiotic degradation via adsorption-photocatalysis and mechanism insight. Environmental Research 214: 114189.
- Li H, Deng F, Zheng Y, Hua L, Qu C, et al. (2019) Visible-light-driven Z-scheme rGO/Bi2S3-BiOBr heterojunctions with tunable exposed BiOBr (102) facets for efficient synchronous photocatalytic degradation of 2-nitrophenol and Cr(VI) reduction. Environmental Science: Nano 6(12): 3670-3683.
- Deng F, Zhang Q, Yang L, Luo X, Wang A, et al. (2018) Dionysiou, Visible-lightresponsive graphene-functionalized Bi-bridge Z-scheme black BiOCl/Bi2O3 heterojunction with oxygen vacancy and multiple charge transfer channels for efficient photocatalytic degradation of 2-nitrophenol and industrial wastewater treatment. Appl Catal B: Environ 238: 61-69.
- Zheng J, Zhang L (2019) Designing 3D magnetic peony flower-like cobalt oxides/g-C3N4 dual Z-scheme photocatalyst for remarkably enhanced sunlight driven photocatalytic redox activity. Chem Eng J 3691: 947-956.
- Tang M, Ao Y, Wang C, Wang P (2020) Facile synthesis of dual Z-scheme g-C3N4/Ag3PO4/AgI composite photocatalysts with enhanced performance for the degradation of a typical neonicotinoid pesticide. Appl Catal B: Environ 2685: 118395.
- Zhang Q, Peng Y, Deng F, Wang M, Chen D (2020) Porous Z-scheme MnO2/Mn-modified alkalinized g-C3N4 heterojunction with excellent Fenton-like photocatalytic activity for efficient degradation of pharmaceutical pollutants. Separation and Purification Technology 246: 116890.
- Smýkalová A, Sokolová B, Foniok K, Matějka V, Praus P (2019) Photocatalytic degradation of selected pharmaceuticals using g-C3N4 and TiO2 Nanomaterials 9(9): 1194.
- Guo N, Zeng Y, Li H, Xu X, Yu H, et al. (2018) Novel mesoporous TiO2@ g-C3N4 hollow core@ shell heterojunction with enhanced photocatalytic activity for water treatment and H2 production under simulated sunlight. Journal of Hazardous Materials 353: 80-88.
- Li K, Gao S, Wang Q, Xu H, Wang Z, et al. (2015) In-situ-reduced synthesis of Ti3+ self-doped TiO2/g-C3N4 heterojunctions with high photocatalytic performance under LED light irradiation. ACS Appl Mater Inter 7: 9023-9030.
- Yao H, Fan M, Wang Y, Luo G, Fei W (2015) Magnetic titanium dioxide based nanomaterials: Synthesis, characteristics, and photocatalytic application in pollutant degradation. J Mater Chem 3: 17511-17524.
- Lu LY, Wang GH, Zou M, Wang J, Li J (2018) Effects of calcining temperature on formation of hierarchical TiO2/g-C3N4 hybrids as an effective Z-scheme heterojunction photocatalyst. Appl Surf Sci 441: 1012-1023.
- Yan SC, Li ZS, Zou ZG (2010) Photodegradation of rhodamine B and methyl orange over boron-doped g-C3N4 under visible light irradiation. Langmuir 26(6): 3894-3901.
- Natarajan TS, Natarajan K, Bajaj HC, Tayade RJ (2011) Energy efficient UV-LED source and TiO2 nanotube array-based reactor for photocatalytic application. Ind Eng Chem Res 50: 7753-7762.
- Natarajan K, Natarajan TS, Bajaj HC, Tayade RJ (2011) Photocatalytic reactor based on UV-LED/TiO2 coated quartz tube for degradation of dyes. Chem Eng J 178: 40-49.
© 2023 Muzammil Anjum. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






