- Submissions

Full Text
Peer Review Journal of Solar & Photoenergy Systems
A Comparison of Different Polymeric Materials for Photocatalytic and Electrochemical Water Splitting: A Review
Amina Khalid and Sohail Nadeem*
Department of Chemistry, School of Science, University of Management and Technology C-II Johar Town Lahore, 54770, Pakistan
*Corresponding author: Sohail Nadeem, Department of Chemistry, School of Science, University of Management and Technology C-II Johar Town Lahore, 54770, Pakistan
Submission: February 15, 2023;Published: March 09, 2023

Volume2 Issue2 March , 2023
Abstract
The development of electrochemical water splitting catalysts have low cost and long-life high-performance generation of H2 and O2. We present the study about the polymeric electrolytes as PU.PANI.FeS2 composed of Polyurethane (PU), Polyaniline (PANI) and iron sulfide (FeS2) used in electrochemical water splitting and H2 and O2 production and now, s study enhances the efficiency of H2 production efficiency. This study may begin a new opening for 𝜋- conjugated polymers, metal-free porphyrin-based crystalline covalent organic polymer, triphenylphosphine skeleton (HCP-PPh3) for sustainable hydrogen and oxygen production. The polypyrene (PPy) conducting polymer enhance photocatalytic activity. Coordination polymers (CPs) have a considerable contribution for use of polymers in noble-metal-free water splitting by electrochemical method by the hydrogen/oxygen evolution reactions (OER/HER). The bimetallic coordination polymers improve the conductivity, and the composites material shows excellent performance with a small Tafel slope and high faradaic efficiency. Whole water splitting produces a current density of 10mA cm-2 at a voltage 1.52V with excellent durability. The polymer of tandem solar cells is also used for electrochemical water splitting which have high power conversion efficiency. The micro patterned Polyurethane Acrylate (PUA) are also efficient in photo electrochemical water splitting. The Polymer Electrolyte Water Electrolysis (PEWE) cell used for high pressure operation. The physical (X-Ray diffraction XRD, Scanning Electron Microscopy (SEM), Energy-Dispersive X-ray Spectroscopy (EDX)) and electrochemical (Cyclic Voltammetry (CV), Linear Sweep Voltammetry (LSV), Fourier transform infrared spectroscopy (FT-IR Electrochemical Impedance Spectroscopy (EIS) methods are used for characterization. The results can help us to future enhance efficiency targets and points towards next generation PEWE materials.
Keywords: HER (Hydrogen Evolution Reaction); OER (Oxygen Evolution Reaction); Polymeric Materials; Splitting
Introduction
Today 98% hydrogen production is generated from fossil energy sources responsible for an emission of O2 per year and less than 1% of the world’s hydrogen (green hydrogen) demand is produced from renewable sources. One of major technology for the production of hydrogen (green hydrogen) is Polymer Electrolyte Water Electrolysis (PEWE) It gives us a way for development of PEWE materials and its components for increasing efficiency and lower cost of hydrogen (green hydrogen production) [1,2]. Water electrolyzes or water splitters can be divided into three categories, Alkaline Electrolysis (AE), Solid Oxide Electrolysis (SOE) and Polymer Electrolyte Membrane (PEM) electrolysis. The overall efficiency is also depending upon electrochemical cell’s temperature and pressure [3,4].
Coordination polymers have attracted considerable attention for use in noble-metal-free electrochemical water splitting, for example, by the Hydrogen or Oxygen Evolution Reactions (HER/OER). Electrochemical water splitting is satisfactory method to produce pure hydrogen. To reduce the over potential for catholic Hydrogen Evolution Reaction (HER) is very difficult in hydrogen production. Catalytic hydrolysis of chemical hydrides includes formic acid, sodium borohydride, and ammonia borane are alternative approaches for hydrogen production [5- 15]. Hydrogen production from water electrolysis significantly depends upon the development of catalysts with high efficiencies. Microporous polymer networks have gained widespread attention due to the unique properties of high surface areas, abundant functional groups and chemical and thermal stability. With coordination of ruthenium catalyst in inner space of the HCP-PPh3 synthesized through cross-linking of PPh3 and benzene. The assynthesized material would be used for hydrogen production from electrochemical water splitting [16].
Inserting units of free-base porphyrins into PPTA networks can result in two- dimensional (2D) porphyrin-based polymers. Porphyrin moieties can enhance interactions and add charge transport properties used in electrocatalytic applications. The use of metal-free porphyrin-based polymer as bifunctional electrocatalysts directly for both HER and OER. The synthesis of a metal-free porphyrin based crystalline 2D porphyrin-based organic polymer called Porphvlar, get from the condensation of terephthaloyl chloride and tetrakis (4-aminophenyl porphyrin, namely H2TAPP) is an effective bifunctional electrocatalysts for OER and HER in neutral solutions [17-24]. The electrochemical reaction of this material is expose through oxidation and reduction conditions to study its catalytic activity, charge transfer ability and stability. Scanning Electron Microscopy (SEM) images were obtained to evaluate the morphology of the synthesized Porphvlar polymer in different size. Figure 1 & 2 shows that the Porphvlar shows a flake-like morphology. The flakes are stacked in layers of nanometers in thickness. This is different to the rod-like morphology of PPTA threads [25]; (Figure 3).
Figure 1:

Figure 2:Synthesizing HCP-PPh3.
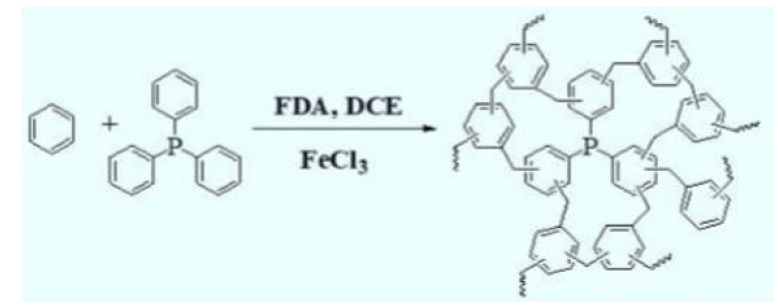
Figure 3:Preparation of phosphorus containing porous polymer.
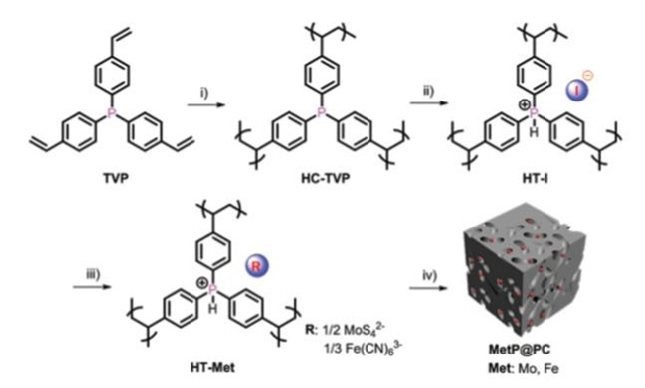
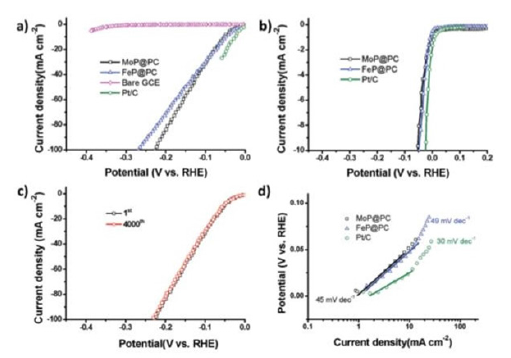
The production of H2 on a large scale Hydrogen Evolution Reaction (HER) and the Oxygen Evolution Reaction (OER) are the two half-cell reactions of electrochemical water splitting. We required highly efficient electrochemical catalysts to proceeds the HER at low overpotentials. We present an efficient approach for preparing porous carbons embedded with transition-metal phosphides (Met P. PCs, where the transition metal center is Met=Mo, Fe) at a large scale through the direct pyrolysis of cationic, phosphorous-based, porous polymer precursors loaded with transition-metal-containing anions under a hydrogen atmosphere. The transition-metal-containing anions and phosphorous-based polymer framework serve as the metal and phosphorous sources for the formation of the Met P. PCs under pyrolytic and reducing conditions. The Met P. PCs process highly specific exhibited electrochemical catalytic activities for the HER in 0.5M H2SO4. MoP. PC (0.24mg cm-2 on a glass carbon electrode) exhibited an overpotential of 51mV at 10mA cm-2 and a Tafel slope of 45mV dec- 1, comparable to those of the commercially available Pt/C catalyst (24mV at 10mA cm-2, 30mV dec-1) [25-30]; (Figure 4).
Figure 4: Tafel slopes of MoP.PC.
There are many other organic polymeric materials which are used in electrochemical materials which a react different in different conditions some are used as catalysts included as Ppy\ZnWO4 nanocomposites [18], triphenylphosphine-hypercrosslinked polymer, tandem polymer cells [14], polypyrrole carbon nanocomposites, Porous organic polymer with carbon nanocomposites [20], PU.PANI.FeS2 nanocomposites with different electrodes [21] and its mechanism of OER and HER mechanism which are given below Figure 5.
Figure 5:Hydrogen Evolution Reaction (HER) Mechanism.
Figure 6:PEM Electrolyzer Working.
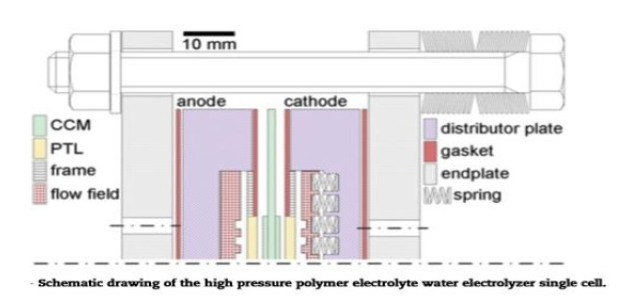
Another way of water splitting through electrochemical way is electrolyzer membrane which is made up of polymeric material as electrode which help them in H2O splitting. The water splitting with electric current the electrolyzers efficiently produce hydrogen [31-40]. The hydrogen production efficiency ηp of Hydrogen H2 will expresses the ratio of the chemical energy is obtained and how much chemical energy must be supplied to the electrolysis cell. π conjugated polymers [2], ZnO based photoelectrode prepared on the micropatterned Polyurethane Acrylate (PUA) [3], polyaniline polymer [9] gives different RHE and voltages and gives different Tafel slopes value and this material is best for electrolyzers. The mechanism of the Polymer Electrolyte Membrane (PEM) is given as Figure 6.
Comparison of Different Results
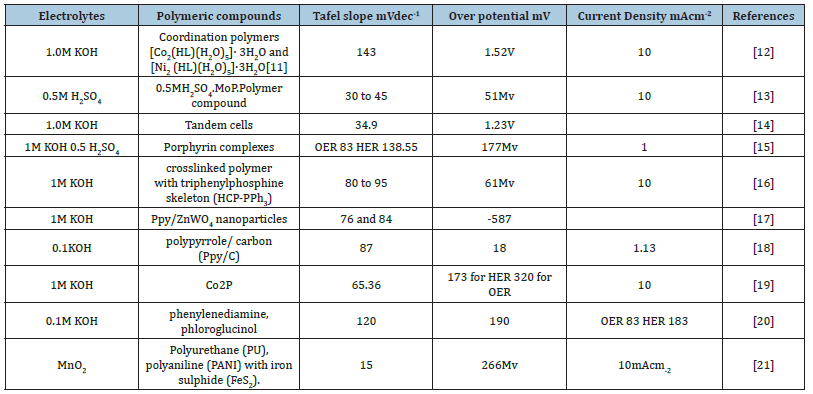
Different polymeric materials are used for water splitting in different medium for its stability and its efficiency. Following is the comparison table and to compare polymeric material through Tafel slopes (mVdec-1) and Over potential (mV), where on the basis of Tafel slopes as the lowest peak the better performance is achieved. Polyurethane (PU), polyaniline (PANI) with iron sulphide (FeS2) is considered to be the best efficiency for HER in electrochemical water splitting [41-51]. However according to over potential 0. 5MH2SO4.MoP. Polymer compounds gives lowest peaks and Tafel slope at 30mVdec-1 is best compound for water splitting. These experiments uses XRD, SEM,TEM and The Temperature of Tafel slope and efficient polymeric material which one is best given through a graph in which it shows in Figure 7 (Table 1).
Figure 7:
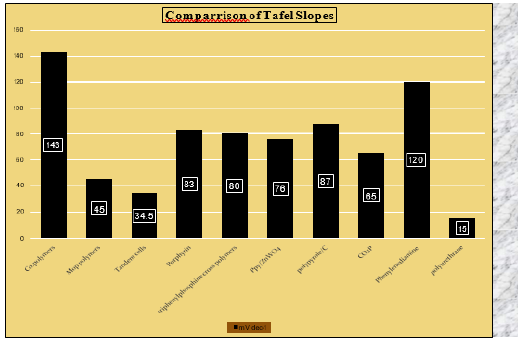
Table 1:
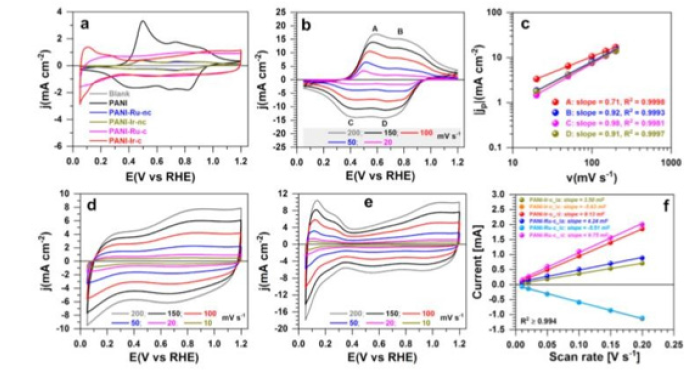
Different Polymer electrolyte water electrolyzer member ionic system is also used at different 9emperature and 50μm thin membranes. It was reveals t that research and development do not need to focus on the development of durable materials for operating at 90 ˚C as an optimal stack temperature can be lower within the relevant current density range, which is limited by efficiency requirements. The polybendezimzole, polythiophenes, polyurethrane acrylte, trimethyl benzyl ammonium group, temperature-based polymers, conjugated polymers, Polyaniline Polymer shows there different RHE vsV value with different temperature at different current densities the water splitting by electrochemical process. Polyaniline polymer and polyurethrane polymers based PEME shows tafel slope at 47 and 21r HER (36Mv RHE at 10Ma cm-2, 21mVdec-1), while PANI-Ru is suitable for OER (1.47V RHE at 10mAcm-2,47mVdec-1) (Figure 8).
Figure 8:V vs RHE value of polyaniline, polyurethrane.
Conclusion
Over potential low peaks and Tafel slope of 30 and 15mV dec- 1 at 154 and 51mV gives the efficient value of water splitting by electrochemical process use polymeric electrocatalysts and KOH and H2SO4 electrolytes. Another way is Polymeric electrolyzer membrane in which different polymeric material is used best one is polyuretherane material which gives Tafel slope at 47mVdec-1 which is lowest in all other materials. Further improvements have been occurring in water splitting.
Acknowledgement
The authors gratefully acknowledge the support received for this research work from the Major Project of Fundamental and Application Research of the Department of Chemistry, School of Science, University of Management and Technology, Lahore, Pakistan and Office of Research Innovation and Commercialization UMT Lahore, Pakistan.
References
- (2019) The future of hydrogen, International Energy Agency (IEA), Paris, France.
- Babic U, Suermann M, Büchi FN, Gubler L, Schmidt TJ (2017) Critical review-identifying critical gaps for polymer electrolyte water electrolysis development. J Electrochem Soc 164: F387-F399 .
- Kopp M, Coleman D, Stiller C, Scheffer K, Aichinger J (2017) Energiepark mainz: Technical and economic analysis of the worldwide largest power-to-gas plant with PEM electrolysis. Int J Hydrog Energy 42(19): 13311-13320 .
- (2018) Hydrogen from renewable power: Technology outlook for the energy tran-sition. Int Renew Energy Agency (IRENA), Masdar City, UAE, pp. 1-50.
- Schmidt O, Gambhir A, Staffell I, Hawkes A, Nelson J, Few S (2017) Future cost and performance of water electrolysis: An expert elicitation study. Int J Hydrogen Energy 42(52): 30470-30492.
- Babic U, Suermann M, Büchi FN, Gubler L, Schmidt TJ (2017) Critical review-identifying critical gaps for polymer electrolyte water electrolysis development. J Electrochem Soc 164(4): F387-F399.
- Ayers KE, Anderson EB, Capuano C, Carter B, Dalton L, et al. (2010) Research advances towards low cost, high efficiency PEM electrolysis. ECS Trans 33(1): 3-15.
- Chandesris M, Medeau V, Guillet N, Chelghoum S, Thoby D, et al. (2015) Membrane degradation in PEM water electrolyzer: Numerical modeling and experimental evidence of the influence of temperature and current density. Int J Hydrogen Energy 40(3): 1353-1366.
- Zhao W, Ai Z, Dai J, Zhang M (2014) Enhanced photocatalytic activity for H2 evolution under irradiation of UV-vis light by Au-modified nitrogen-doped TiO2. PLoS One 9(8): e103671.
- Chu D, Masuda Y, Ohji T, Kato K (2010) Formation and photocatalytic application of ZnO nanotubes using aqueous solution. Langmuir 26(4): 2811-2815.
- Jiang Q, Wu ZY, Wang YM, Cao Y, Zhou CF, et al. (2006) Fabrication of photoluminescent ZnO/SBA-15 through directly dispersing zinc nitrate into the as-prepared mesoporous silica occluded with template. J Mater Chem 16(16): 1536-1542.
- Pita K, Baudin P, Ving Vu Q, Aad R, Couteau C, et al. (2013) Annealing temperature and environment effects on ZnO nanocrystals embedded in SiO2: A photoluminescence and TEM study. Nanoscale Res Lett 8: 517.
- Fujishima A, Honda K (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature 238(5358): 37-38.
- Schlapbach L, Zuttel A (2001)Hydrogen-storage materials for mobile applications. Nature 414: 353-358.
- Hirscher M, Autrey T, Orimo SI (2019) Hydrogen energy. Chem Phys Chem 20(10): 1157.
- Bicakova O, Straka P (2012) Production of hydrogen from renewable resources and its effectiveness. Int J Hydrogen Energy 37(16): 11563-11578.
- Muradov N (2001) Hydrogen via methane decomposition: An application for decarbonization of fossil fuels. Int J Hydrogen Energy 26(11): 1165-1175.
- Andrews J, Shabani B (2012) Re-envisioning the role of hydrogen in a sustainable energy economy. Int J Hydrogen Energy 37(2): 1184-1203.
- Sata T, Tsujimoto M, Yamaguchi T, Matsusaki K (1996) Change of anion exchange membranes in an aqueous sodium hydroxide solution at high temperature. J Membr Sci 112(2): 161-170.
- Vega JA, Chartier C, Mustain WE (2010) Effect of hydroxide and carbonate alkaline media on anion exchange membranes. J Power Sources 195(21): 7176-7180.
- Nikolov I, Darkaoui R, Zhecheva E, Stoyanova R, Dimitrov N, et al. (1997) Electrocatalytic activity of spinel related cobalties MxCo3-xO4 (M= Li, Ni, Cu) in the oxygen evolution reaction. J Electroanal Chem 429(1-2): 157-168.
- Ursua A, Gandia LM, Sanchis P (2012) Hydrogen production from water electrolysis: Current status and future trends. Proc IEEE 100(2): 410-426.
- Millet P, Grigoriev S (2013) Water electrolysis technologies. In: Gandia LM, Arzamendi G, Dieguez PM (Eds.), Renewable Hydrogen Technologies: Production, Purification, Storage, Applications and Safety. Elsevier, Amsterdam, Netherlands, pp. 19-41.
- Barnhart CJ, Dale M, Brandt AR, Benson SM (2013) The energetic implications of curtailing versus storing solar-and wind-generated electricity. Energy Environ Sci 6: 2804-2810.
- Stamenkovic VR, Strmcnik D, Lopes PP, Markovic NM (2016) Energy and fuels from electrochemical interfaces. Nat Mater 16(1): 57-59.
- Wang X, Li W, Xiong D, Petrovykh DY, Liu L (2016) Bifunctional nickel phosphide nanocatalysts supported on carbon fiber paper for highly efficient and stable overall water splitting. Adv Funct Mater 26(23): 4067-4077.
- De Faria LA, Boodts JFC, Trasatti S (1996) Electrocatalytic properties of ternary oxide mixtures of composition Ru3Ti(0.7x)CexO2: Oxygen evolution from acidic solution. J Appl Electrochem 26: 1195-1199.
- Liao PQ, Shen JQ, Zhang JP (2018) Metal-organic frameworks for electrocatalysis. Coordin Chem Rev 373: 22-48.
- Wang W, Xu X, Zhou W, Shao Z (2017) Recent progress in metal- organic frameworks for applications in electrocatalytic and photocatalytic water splitting. Adv Sci 4(4): 1600371.
- Wang HF, Chen L, Pang H, Kaskel S, Xu Q (2020) MOF-derived electrocatalysts for oxygen reduction, oxygen evolution and hydrogen evolution reactions. Chem Soc Rev 49(5): 1414-1448.
- Kibsgaard J, Jaramillo TF (2014) Molybdenum phosphosulfide: An active, acid-stable, earth-abundant catalyst for the hydrogen evolution reaction. Angew Chem Int Ed Engl 53(52): 14433.
- Bahro D, Koppitz M, Mertens A, Glaser K, Mescher J, et al. (2015) Understanding the external quantum efficiency of organic homo-tandem solar cells utilizing a three-terminal device architecture. Adv Energy Mater 5(22): 1501019.
- Chang BY, Park SM (2010) Electrochemical impedance spectroscopy. Annu Rev Anal Chem 3(1): 207-229.
- Lasia A (2002) Electrochemical impedance spectroscopy and its applications. In: Conway BE, Bockris JO, White RE (Eds.), Modern Aspects of Electrochemistry, Springer, Boston, MA, USA, pp. 143-248.
- He W, Zhang F, Li H (2011) Active and reusable Pd(ii) organometallic catalyst covalently bonded to mesoporous silica nanospheres for water-medium organic reactions. Chem Sci 2(5): 961-966.
- Ge C, Jiang P, Cui W, Pu Z, Xing Z, et al. (2014) Shape-controllable synthesis of Mo2C nanostructures as hydrogen evolution reaction electrocatalysts with high activity. Electrochim Acta 134: 182-186.
- Zhang Y, Liu Y, Ma M, Ren X, Liu Z, et al. (2017) A Mn-doped Ni2P nanosheet array: An efficient and durable hydrogen evolution reaction electrocatalyst in alkaline media. Chem Commun 53(80): 11048-11051.
- Park S, Shao Y, Liu J, Wang Y (2012) Oxygen electrocatalysts for water electrolyzers and reversible fuel cells: Status and perspective. Energy Environ Sci 5(11): 9331-9344.
- Bhat KS, Barshilia HC, Nagaraja HS (2017) Porous nickel telluride nanostructures as bifunctional electrocatalyst towards hydrogen and oxygen evolution reaction. Int J Hydrogen Energy 42: 24645e55.
- Gong M, Dai H (2015) A mini review of NiFe-based materials as highly active oxygen evolution reaction electrocatalysts. Nano Research 8: 23-39.
- Liang Y, Li Y, Wang H, Zhou J, Wang J, et al. (2011) Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat Mater 10(10): 780.
- Jiang Z, Jiang ZJ, Maiyalagan T, Manthiram A (2016) Cobalt oxidecoated N-and B-doped graphene hollow spheres as bifunctional electrocatalysts for oxygen reduction and oxygen evolution reactions. J Mater Chem A 4(16): 5877-5889.
- Gu MX, Kou Y, Qi SC, Shao MQ, Yue MB, et al. (2019) Highly dispersive cobalt oxide constructed in confined space for oxygen evolution reaction. ACS Sustainable Chem Eng 7(2): 2837-2843.
- Ullah N, Shah SA, Xie M, Rasheed HU, Oluigbo CJ, et al. (2019) 3D graphene decorated with hexagonal microcoin of Co(OH)2: a competent electrocatalyst for hydrogen and oxygen evolution reaction. Int J Hydrogen Energy 44(29): 14770-14779.
- Bashyam R, Zelenay P (2006) A class of non-precious metal composite catalysts for fuel cells. Nature 443(7107): 63-66.
- Turner JA (2004) Sustainable hydrogen production. Science 305(5686): 972-974.
- Faber MS, Jin S (2014) Earth-abundant inorganic electrocatalysts and their nanostructures for energy conversion applications. Energy Environ Sci 7: 3519-3542.
- Peng Y, Wong WK, Hu Z, Cheng Y, Yuan D, et al. (2016) Room temperature batch and continuous flow synthesis of water-stable covalent organic frameworks (COFs). Chem Mater 28: 5095-5101.
- Puthiaraj P, Lee YR , Zhang S, Ahn WS (2016) Triazine-based covalent organic polymers: Design, synthesis and applications in heterogeneous catalysis. Mater Chem A 4: 16288-16311.
- Yang M, Long X, Li H, Chen H, Liu P (2019) Porous organic-polymer-derived nitrogen-doped porous carbon nanoparticles for efficient oxygen reduction electrocatalysis and supercapacitors. ACS Sust Chem Eng 7: 2236-2244.
- Wang N, Burugapalli K, Song W, Halls J, Moussy F, et al. (2013) Tailored fibro-porous structure of electrospun polyurethane membranes, their size-dependent properties and trans-membrane glucose diffusion. Journal of Membrane Science 427: 207-217.
© 2023 Sohail Nadeem. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






