- Submissions

Full Text
Peer Review Journal of Solar & Photoenergy Systems
Highly Crystalline Surface Supported Metal Organic Thin Film Materials Based Heterojunctions for Triplet-Triplet Annihilation Upconversion
Shargeel Ahmad*
State Key Laboratory of Fine Chemicals, China
*Corresponding author: Shargeel Ahmad, Institute of Artificial Photosynthesis (IAP), State Key Laboratory of Fine Chemicals, China
Submission: October 30, 2018;Published: November 21, 2018

Volume1 Issue2 November 2018
Opinion
We know that finding highly efficient, cheap and versatile renewable energy conversion materials is the thirst of this century. Harnessing the idea of triplet-triplet annihilation up conversion (TTA UC) requires a smart hybrid material overcoming required distance for smooth and efficient triplet energy transfer (TEnT) [1- 3]. However, the TTA UC process is the one of the best wavelength shift methodology in which the two low energy photons ( ) having high wavelength are absorbed and transformed into one high energy photons ( ) with low wavelength via Dexter type energy transfer mechanism [3,4]. In our previous demonstration we have reported the triplet energy transfer between PtOEP (PtOEP=Pt (II) octaethylporphine) as sensitizer and Zn-perylene SURMOF as acceptor in acetonitrile solution [5] by making solid liquid interface and surface modifications. Here we will put a novel idea of suing solid-solid interface by making SURMOF-SURMOF heterojunction to study TTA UC.
The TTA UC has been studied using variety of materials to enhance the contemporary demands of solar energy [1,6,7]. Moreover, notable efforts have been made to utilize the modern surface-anchored metal-organic frameworks (SURMOFs) materials in gas separation [8] electronics [9-11] CO2 reduction [12-15] water splitting [16,17] photovoltaic [18-20] and most recently in TTAUC system [3,5] due to its controlled growth orientation, tunable pore size and highest crystallinity. Although, the obtained results in the literature and our studied systems were encouraging but the triplet energy had to cross the solid liquid interfaces. Moreover, the random orientation of photosensitizer which was dissolved in the solution could also hinder the transfer of triplet energy in the photoelectrochemical cell [5].
It has been reported that the Zn (II) tetraphenylpophyrin molecules have both and bond between N atom and Zn+2 transition metal. The Zn+2 and N atom have coordination due to d electrons which strengthens the (T1←S1) transition [21]. As a matter of fact, the Zn (II) tetraphenylpophyrin photosensitizer can also effectively utilize the long-lived S2 states (1.5 and 2.4ps) with strong transition (S2←S0) followed by hopping process with S2 excitation energy which needs the emitter of higher energy level [22,23].
Moreover, the blue emitter-perylene molecule has lower energy level which favors the triplet energy transfer (TEnT) followed by triplet-triplet annihilation mechanism from sensitizer and the exchange of triplet energy with acceptor annihilating the triplets for the formation of singlets to generating the blue light with high energy. In this work we will introduce the formation of heterojunction with Zn (II) tetraphenylpophyrin molecules as sensitizer and 3,9-perpylenedicarboxylic acids as acceptor which will be used for triplet-triplet annihilation up conversion (TTA UC) shown in Figure 1.
Figure 1:Schematic illustration (A) is the SURMOFSURMOF heterostructure on top of coarse glass/FTO (B) the controlled growth of SURMOF-SURMOF heterostructure which favors the conversion of green light into blue light via TTA UC. Pophyrin: Zn (II) tetraphenylpophyrindicarboxylic acid.
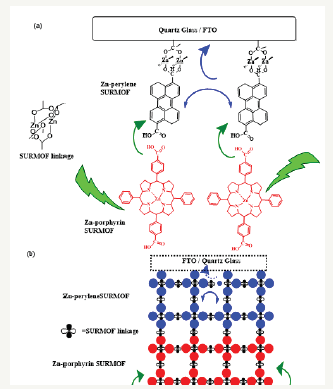
Experimental Section
Preparation of substrates
The quartz glass/FTO glass (SOLARONIX, Switzerland) substrates were cleaned in acetone for approximately ten minutes in an ultrasonic bath then these are treated with plasma under O2 for nearly thirty minutes to generate a surface with -OH (hydroxyl groups).These cleaned substrates were used instantaneously to grow SURMOF.
Preparation of Zn-perylene SURaMOF
For preparation of SURMOF we have used the similar strategy mentioned in chapter three. Here the different substrate such as coarse glass and FTO was used. Moreover, we additionally found that concentration of perylene could be adjusted between 20 to 40μM which is dependent upon the optimized growth of SURMOF.
Preparation of Zn-porphyrin SURMOF and its heterojunction
SURMOF of Zn(II) metalloporphyrin were fabricated using well established highly throughput automated spray system Briefly, a concentration of 20μM Zn(II)metalloporphyrin’s in ethanol (spray time: 25s, waiting time: 35s) and a concentration of 1mM zinc acetate in ethanol (spray time: 15s, waiting time: 35s) were one by one sprayed onto the FTO/Quartz substrates in a layer-bylayer fashion using N2 as a carrier gas (0.2mbar). In between, pure ethanol was used for rinsing to get rid of the unreacted species from the surface (rinsing time: 5s). The thickness of the sample was controlled by the number of deposition cycles.
The SURMOF-SURMOF heterojunction was formed by firstly growing the 20 cycles of Zn-perylene SURMOF and on top of it 20 more cycles of Zn(II) metalloporphyrin SURMOF was added to make heterojunctions. Moreover, the formation of heterojunction which is described in the literature [4] has also been followed.
Preparation of Zn-perylene SURMOF +Zn-porphyrin SURMOF heterostructure for TTA UC
First of all, 40mg/ml PMMA (poly methyl (methacrylate) was prepared in the acetonitrile solution. Then as prepared MOF thin film material consisting of FTO/Glass-Zn-perylene SURMOF+Znporphyrin SURMOF were immersed into the well mixed acetonitrile solution of PMMA which was degassed with N2 for half an hour. The heterostructure was characterized for triplet-triplet annihilation up conversion. Using laser light source.
XRD Characterizations
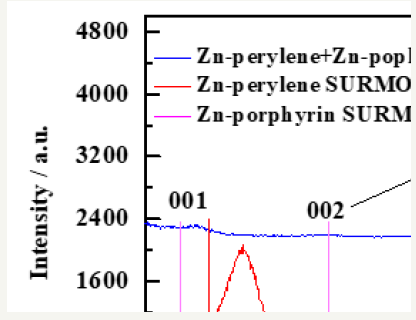
The SURMOF-SURMOF heterojunction has been characterized which showed (001) & (002) preferred orientation Figure 2. These results were compared with the reported studies [4].
Figure 2:XRD of Zn-porphyrin (red); Zn-perylene (red) and Zn-perylene+Zn-porphyrin heterostructure.
Detail Studies of SURMOF-SURMOF Heterojunction and Its Applications
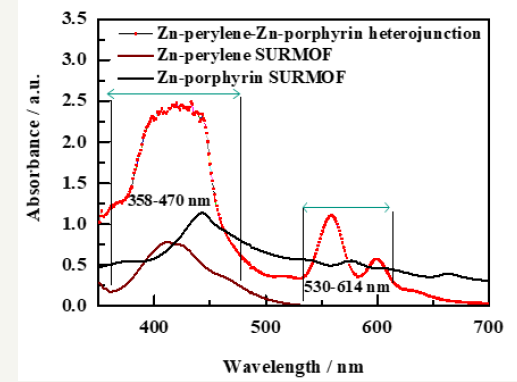
Comparative analysis of the ultraviolet-visible (UV-vis) spectrum of Zn-perylene SURMOF, Zn-porphysin SURMOF and Zn-perylene- Zn-porphyrin heterojunction is being shown in Figure 3. The UV¬- vis spectrum of Zn-perylene alone SURMOF range from 358nm to 470nm (in brown) which is also compared with the solution of free perylene dicarboxylic [24] acids indicating a blue shift in MOF thin film sample. The UV-vis of Zn-porphyrin shows a Sorret Band at ~440nm and two Q bands between 530nm to 614nm. The Zn(II) tetraphenylpophyrin [17,25] molecule shows two Q bands which are different from free base porphyrin generating four Q bands because Zinc+2 ion coordination with porphyrin molecule changes the symmetry [17] of the former molecule The combined UV vis of Zn-perylene SURMOF and Zn-porphyrin SURMOF heterostructure overlaps with all the bands of both MOF thin films shown in Figure 3 (red).The merging of all the bands in SURMOF heterostructure is very important for efficient absorption of green light and its conversion into blue light shown in Figure 3.
Figure 3:UV-vis spectra of Zn-perylene SURMOF(brown)-Zn-porphyrin SURMOF (black) and Zn-perylene SURMOF-Zn-porphyrin SURMOF heterojunctions (red).
The SEM characterization of SURMOF-SURMOF heterojunction shows that the first 20 cycles of highly crystalline Zn-perylene MOF thin film have grown the ~200nm thick film Moreover, the addition of Zn-porphyrin SURMOF Zn-perylene SURMOF could grow more ~200nm thick shown in Figure 4. The phenomenon of TTA UC has been studied with SURMOF-SURMOF heterojunction which showed enhanced energy shown in Figure 5. This give us the idea that MOF thin film based highly crystalline and versatile materials is very useful for energy conversion devices.
Figure 4:Scanning electron microscope showing the thickness of SURMOF-SURMOF heterostructure.
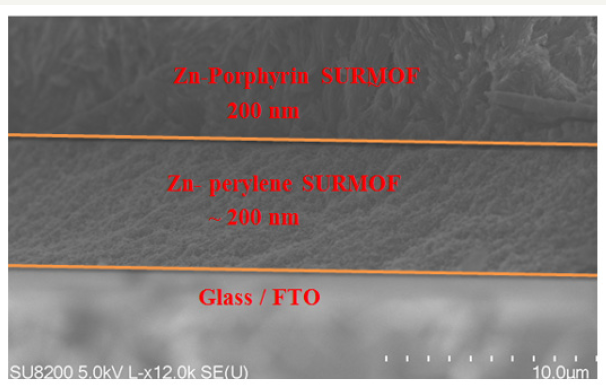
Figure 5:Demonstration of TTA UC with SURMOF-SURMOF heterojunctions. (b) The SURMOF-SURMOF based intensity dependent behavior under 532nm green light Irradiation.

The obtained quantum yield efficiency of Zn-perylene SURMOF+Zn-porphyrin SURMOF heterostructure is 0.182%. Following the same method of calculation mention in the reported literature we found that the calculated value is consistent with the literature values [18]. However, it is highly recommended to use the heterojunction for future dye sensitized solar cell devices. Summing up, the MOF thin film based smart hybrid materials can be used for triplet-triplet annihilation upconversion. This functional material can be effectively used for the future energy conversion devices. The point of view is that a prototype dye sensitized solar cell device can be fabricated with highly crystalline MOF thin film. Moreover, it has been demonstrated that the photocurrent can be significantly enhanced due to triplet-triplet annihilation up-conversion. Further efforts in such direction may open the new avenues for exploring more MOF thin film materials for solar energy conversion devices.
References
- Hill SP, Dilbeck T, Baduell E, Hanson K (2016) Integrated photon upconversion solar cell via molecular self-assembled bilayers. ACS Energy Letters 1(1): 3-8.
- Monguzzi A, Tubino R, Hoseinkhani S, Campione M, Meinardi F (2012) Low power, non-coherent sensitized photon up-conversion: modelling and perspectives. Physical Chemistry Chemical Physics 14(13): 4322- 4332.
- Simon YC, Weder C (2012) Low-power photon upconversion through triplet-triplet annihilation in polymers. Journal of Materials Chemistry 22(39): 20817-20830.
- Oldenburg M, Turshatov A, Busko D, Wollgarten S, Adams M (2016) Photon upconversion at crystalline organic-organic heterojunctions. Adv Mater 28(38): 8477-8482.
- Ahmad S, Liu J, Gong C, Zhao J , Sun L (2018) Photon up-conversion via epitaxial surface-supported metal-organic framework thin films with enhanced photocurrent. ACS Appl Mater Interfaces 1(2): 249-253.
- Hill S P, Banerjee T, Dilbeck T, Hanson K (2015) Photon upconversion and photocurrent generation via self-assembly at organic-inorganic interfaces. J Phys Chem 6(22): 4510-4517.
- Simpson C, Clarke TM, Macqueen RW, Cheng YY, Trevitt AJ (2015) An intermediate band dye-sensitised solar cell using triplet-triplet annihilation. Physical Chemistry Chemical Physics 17(38): 24826- 24830.
- Wang Z, Knebel A, Grosjean S, Wagner D, Bräse S (2016) Tunable molecular separation by nanoporous membranes. Nature Communications 7: 13872-13879.
- Wu G, Huang J, Zang Y, He J, Xu G (2016) Porous field-effect transistors based on a semi conductive metal-organic framework. Journal of the American Chemical Society 139(4): 1360-1363.
- Liu H, Chang L, Chen L, Li Y (2016) Nanocomposites of platinum/ metal-organic frameworks coated with metal-organic frameworks with remarkably enhanced chemoselectivity for cinnamaldehyde hydrogenation. Chemcatchem 8(5): 946-951.
- Wang Z, Nminibapiel D, Shrestha P, Liu J, Guo W, et al. (2016) Resistive switching nanodevices based on metal-organic frameworks. Chem Nano Mat 2(1): 67-73.
- Kornienko N, Zhao Y, Kley CS, Zhu C, Kim D, et al. (2015) Metal-organic frameworks for electrocatalytic reduction of carbon dioxide. Journal of the American Chemical Society 137(44): 14129-14135.
- Choi KM, Kim D, Rungtaweevoranit B, Trickett CA, Trese J et al. (2017) Plasmon-enhanced photocatalytic CO2 conversion within metal-organic frameworks under visible light. Journal of the American Chemical Society 139(1): 356-362.
- Ye L, Liu J, Gao Y, Gong C, Addicoat M, et al. (2016) Highly oriented MOF thin film-based electrocatalytic device for the reduction of CO2 to CO exhibiting high faradaic efficiency. Journal of Materials Chemistry A 4(40): 15320-15326.
- Wang M, Liu J, Guo C, Gao X, Gong C et al. (2018) Metal-organic frameworks (ZIF-67) as efficient cocatalysts for photocatalytic reduction of CO2: the role of morphology effect. Journal of Materials Chemistry A 6(11): 4768-4775.
- Miner EM, Dincă M (2016) Metal-organic frameworks: evolved oxygen evolution catalysts. Nature Energy 1(12): 16186.
- Hod I, Deria P, Bury W, Mondloch JE, Kung CW et al. (2015) A porous proton-relaying metal-organic framework material that accelerates electrochemical hydrogen evolution. Nature Communications 6: 8304- 8313.
- Liu JX, Zhou WC, Liu JX, Howard I, Kilibarda G et al. (2015) Photoinduced charge-carrier generation in epitaxial MOF thin films: high efficiency as a result of an indirect electronic band gap? Angew Chem Int Ed 54(25): 7441-7445.
- Liu J, Zhou W, Liu J, Fujimori Y, Higashino T, et al. (2016) A new class of epitaxial porphyrin metal-organic framework thin films with extremely high photocarrier generation efficiency: promising materials for allsolid- state solar cells. Journal of Materials Chemistry A 4(33): 12739- 12747.
- Ahrenholtz SR, Epley CC, Morris AJ (2014) Solvothermal preparation of an electrocatalytic metalloporphyrin MOF thin film and its redox hopping charge-transfer mechanism. Journal of the American Chemical Society 136(6): 2464-2472.
- Sakamoto R, Hoshiko K, Liu Q, Yagi T, Nagayama T et al. (2015) A photofunctional bottom-up bis (dipyrrinato) zinc (II) complex nanosheet. Nat Commun 6: 6713.
- Sugunan SK, Tripathy U, Brunet SM, Paige MF, Steer RP (2009) Mechanisms of low-power noncoherent photon upconversion in metalloporphyrin-organic blue emitter systems in solution. The Journal of Physical Chemistry A 113(30): 8548-8556.
- Duvanel G, Grilj J, Vauthey E (2013) Ultrafast long-distance excitation energy transport in donor-bridge-acceptor systems. J Phys Chem A 117(5): 918-928.
- Joblin C, Salama F, Allamandola L (1999) Absorption and emission spectroscopy of perylene (C20H12) isolated in Ne, Ar, and N2 matrices. Journal of Chemical Physics 110(15): 7287-7297.
- Wang S, Peng Y, Zhang C, Li Y, Liu C (2016) Synthesis of zinc porphyrins and effect of peripheral substituents on the coordination reaction.
© 2018 Shargeel Ahmad. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






