- Submissions

Full Text
Peer Review Journal of Solar & Photoenergy Systems
Light Energy Conversion and Storage by Phase Change Materials
Asit Baran Samui*
Institute of Chemical Technology, India
*Corresponding author: Asit Baran Samui, Institute of Chemical Technology, Nathalal Parekh Marg, Matunga, Mumbai 400019, India, Email:asit_samul@yahoo.com
Submission: September 10, 2018;Published: October 16, 2018

Volume1 Issue2 October 2018
Abstract
There are constant efforts to minimize the energy load by using phase change materials (PCM). The development of PCM has reached a stage where the applications can be planned with desirable properties. Together with development of PCM strategies one parallel area is slowly growing, which is the use of PCM and its nanocomposites for tapping solar energy in addition to the existing photovoltaic solar cells. There are some efforts to commercialize the technologies. More and more efforts are being done to store the thermal energy by using molecular strategies. The present paper discusses some of the developments in the area of tapping light energy for generation of heat and its storage.
Introduction
The foot prints of carbon and greenhouse gases by the use of the conventional resources leads to create health, environmental and other socio- economic problems throughout the world [1]. The waste heat is available everywhere, from industrial processes, to solar heat, and even the heat coming out of vehicles. The heat just wasted can be recycled by using phase change materials. Thermal energy storage offers enormous potential for a wide range of energy technologies. Solar energy with abundant nature is the only renewable energy source that can replace fossil fuels to maximum possible extent [2-5].
As a result, the research the development of sustainable solar energy conversion and storage technologies has attracted constantly growing interest. Among other factors, the efficiency of energy conversion and storage depends on the structure and property of the materials used [6,7]. Various prospective nanomaterials have been studied for their energy conversion efficiency and storage of solar irradiation [8,9] Photoinduced nanocarbons, driven by sunlight, are one of the most attractive materials in modern chemistry [10-12].
There are efforts to utilize the PCM as an energy storage system and the energy is harvested from sunlight. Excess heat from sunlight is already utilized. The use of PCM will make the system store the abundant energy more efficiently.
Photo-Thermal Energy Conversion
The conversion of abundant solar energy in to usable form of energy motivated many research activities including utilization phase change materials, which can store energy during exposure to sunlight and utilized at night. Many researchers have attempted to establish the conversion of light energy to heat and its storage.
PCM Performance Under Sunlight Irradiation
Single walled carbon nano tube (SWNT) based systems
The SWNTs are used as a nanoscale photon antenna, which acts as an effective photon capturer and molecular heater of the light-to-heat conversion process. The optical absorptions of SWNTs are due to resonant band-to-band transitions [13] and π-plasmon excitation [14-16] PEG also absorbs the near-infrared light of the solar irradiation [17]. Also, the SWNTs form SWNT/PCM composites having high thermal conductivity that shorten the heat storage and release time [18,19]. Obviously, this composite is able to harvest visible light and convert it to thermal energy much more efficiently as compared to the traditional organic PCM for latent heat thermal energy storage [20,21]
During solar irradiation, the SWNTs of the SWNT/PCM composites do rapid and UV-vis sunlight-harvesting and lightthermal conversion, while the generated thermal energy is stored in the PCMs by a form-stable phase transition with a high energy storage density [22]. These sunlight-driven SWNT/PCM composites are qualified for important potential application in renewable and clean energy sources.
Single walled nanotube (SWNT) is blended with polyethylene glycol 10000 (PEG 10000)-co-N, N′-dihydroxy ethyl aniline to be used as a form-stable polymer PCM (SWNT/PCM) composite [23].
The SWNT/PCM composites exhibit several smart features, such as latent heat storage with high energy storage density, excellent flexibility, high light-to-heat and energy storage efficiency (η>0.84). Above features advocate the use of SWNT/PCM composites for smart clothing or leather through fabric blending or wire drawing. Also, the composites, having strong near-infrared absorbance characteristics and high energy storage density, have a potential application in military stealth. Carbon nanotube sponge (CNTS) can be used as a porous scaffold to encapsulate paraffin wax (PW), which makes an electrically conductive composite with enhanced phase change enthalpy and thermal conductivity (Figure 1) [24]. This composite has practical importance due to its ability for efficiently storing thermal energy from electro heat conversion or light absorption.
Figure 1:Multifunctional phase change composite of paraffin wax and CNT sponge for energy storage from solar radiation [24].
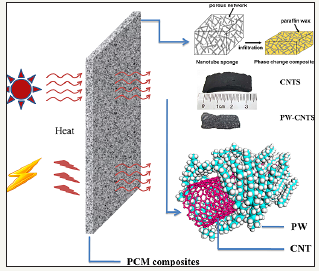
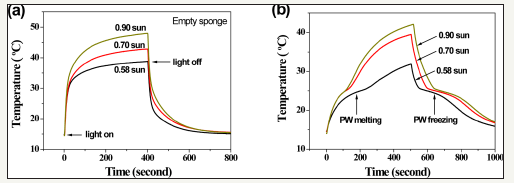
The presence of nanotubes enhances photon absorption, which heats the PW in the composite. Under simulated solar illumination at controlled intensities of 58, 70, and 90mW/cm2 the surface temperature of a blank CNTS increases abruptly from 15 °C to more than 30 °C that reaches equilibrium over a period of 400s. When a PW/CNTS composite (87wt.% loading) is exposed to light, phase change occurs at temperatures close to 25 °C. under all light intensities, which can be seen from the reduced slope and inflection point in each curve (Figure 2) [24]. The heat is released from the composite during the cooling of PW after turning off the light. Composites have energy storage efficiencies of 40% to 60% which increase with higher light intensity.
Figure 2:Thermal energy storage by simulated sunlight absorption. (a) Temperature evolution curves of an empty sponge illuminated by simulated sunlight (AM 1.5) at intensities of 58,70, and 90mW/cm2, showing abrupt increase and decrease of temperature when light turns on and off. (b) Temperature evolution curves of a PW/CNTS composite under the same illumination conditions, showing that PW melting and freezing occur during the heating and cooling processes [24].
Graphene based systems
The GO sheets can easily be functionalized via covalent linkage to control its physical and chemical properties for different applications [25]. Thus, oleyl amine functionalized reduced graphene oxide (OA-rGO), used for shape stabilization, absorbs palmitic acid to make shape stabilized composite [26]. The connected graphene network provides nuclei for the heterogeneous nucleation and crystallization of PA and also provides enhanced heat transfer while maintaining excellent shape stability. The black surface of OA-rGO facilitates capturing the photon energy and heating the PA molecules, which store the thermal energy via phase transition [27]. Similar to heating rate, the cooling rate for PG composites is higher than for pure PA due to the enhanced thermal conductivity of the composites (Figure 3) [26].
A typical nanocomposite shape stabilized PCM [Polyethylene glycol/graphene oxide, (PEG/GO)] absorb energy during the light radiation, which raises its temperature [28]. When the temperature reaches the melting point of PEG, an inflection point appears that indicates the onset of phase change. After removal of the lamp at the temperature of about 60 °C, it was allowed to cool and release heat. However, there is slight rise in temperature, which is accounted for the released energy and there is decrease of temperature after that. The curves can be divided into two parts which represent melting and crystallization progress respectively. Further insight in to the process reveals that the pristine PEG melts much slowly and crystalize very fast. The introduction of GO into the composite enhances the absorption of the light, which ensures fast melting process. During the crystallization part, the released energy raises the sample’s temperature due to re-absorption of energy by PCMs composites. Thus, the thermal absorptivity is enhanced to considerable extent due to small amount of GO and also, it provides a better performance in photo-energy absorption and formstability. The overall effect is the better utilization of energy.
Figure 3:Obtained temperature of Pure PA and PG composites (light irradiation, ambient temperature 28 °C) [26].

Hybrid graphene aerogels (HGA) comprising GO and graphene nanoplatelets (GNP) were prepared and introduced into polyethylene glycol (PEG) via vacuum impregnation to obtain composite PCMs having superior properties along with the ability for light-to-heat energy storage [29]. In order to optimize its solar energy storage and release, the light-to-heat energy conversion experiments was carried out on the composite PCM using solar irradiation. The general characteristics remains similar to that observed by Xiong et al. [26]. As compared to PEG/HGA composite PCMs, the pure PEG has a white surface that would reflect the irradiating light, which meant that the PEG could not reach its melting point and realize the solar energy storage. Thus, the composite PCMs based on HGA had better performance in light-to-heat energy conversion. The lightto- heat and energy storage efficiency of the composite PCMs can be calculated by using photothermal calculation [30]. The energy conversion efficiency of the composite PCMs varies in the range of 80-90%.
Graphene foam (GF) fabricated by CVD is a monolith of a graphene 3D network, which paves the way for phonon transport with small resistance through the high-quality and continuous CVDgrown graphene building blocks [31]. This continuous GF monolith with a high basal-plane solid thermal conductivity of graphene is blended with a PCM to act as a thermal conductive filler with ability for phonon transport [32].
However, the pore size of GF being hundreds of micrometers with low density the thermal resistance of the heat transfer from the PCM inside the large pore to the GF strut walls is obviously large. Therefore, the improvement of the thermal conductivity of the composite PCM is done by growing a network of CNTs inside the pores [33]. In another approach the conductive path of GF is increased by filling the large pores of GF with a denser interconnected hollow graphene network (HGN). The obtained GF filled with the HGN [hierarchical GF (HGF)] is integrated with paraffin wax (PW) via vacuum impregnation to obtain a PW/ HGF composite PCM. The HGF functions as a photon antenna that realizes photon capture and light-to-thermal energy conversion. Therefore, with solar irradiation the PW/HGF composite PCM can harvest solar energy and convert it to thermal energy, which is then stored in the composite PCM with high energy storage density. The PW/GF and PW/HGF composite PCMs absorb over the whole UV–vis–NIR range, while the pure PW shows no absorption peak in the visible-light range. The black color of the HGF allows it ideal light absorbance, which enhanced the photo-absorption of the composite PCMs [34]. The thermal energy storage efficiencies of PW/GF and PW/HGF composite PCMs are high at around 88-89%.
Phase Change Material Based Domestic Solar Cooking System
Solar thermal energy can be stored during sunshine hours by using various techniques which can be used during off sunshine hours effectively for useful purposes. Kenisarin & Mahkamov [33] conducted various studies using different phase change materials which are useful for active and passive solar space heating and cooling and solar cooking applications [35]. The PCM developed for solar application was based on tube, filled with PCM, which was exposed to sunlight and the molten PCM is used for cooking by designing hot plate [36]. The heat transfer enhancement in latent heat thermal energy storage was attempted by Merlin by using conductive structures like finned exchangers, graphite powder and expanded natural graphite (ENG) [37]. Practically, heat exchanger tube and the outer annular of the heat exchanger, filled with PCM, make the system. The PCM incorporated in ENG matrix is the best storage configuration for industrial purposes with the overall heat transfer coefficient around 3000Wm-2K-1 and the thermal conductivity 100 times more than that of PCM alone. The solar cooking system comprising a solar concentrator in the form of parabolic dish and a receiver or cooking pot, which is placed at the focal point of the parabolic dish, performs well with high absorption of solar radiation [38].
PCMS Used in Photovoltaic Modules Thermal Management
It is known that part of the solar energy absorbed by a photovoltaic cell is converted into electrical energy while the rest is wasted as heat that increases in the temperature of the solar cell. In fact, the operating temperature of a photovoltaic device could reach as high as 80 °C at high solar radiation intensity. The opencircuit current increases at elevated temperature but is associated with a decrease in open circuit voltage. The overall effect of changes in current and voltage causes a reduction in the output power of PV cells. Jiang et al. [39] observed that the maximum output power of PV modules under 1000Wm-2 could decrease from 240W to196W as the temperature is raised from 0 °C to 75 °C [40]. The PCMbased thermal management systems are considered to be effective in controlling the temperature of photovoltaic modules and reducing the loss of photoelectric conversion efficiency. According to the prediction from simulation, using realistic one-day weather conditions, the maximum temperature of the front surface of the system with PCMs remains below 34 °C, which helped in achieving high electrical conversion efficiency.
Storage of Energy by PCM Having Molecular Interaction with Photoactive Molecule
The sensitivity of phase-transition to the temperature of the surrounding prevents the long-term storage of latent heat without loss to the environment remains a challenge [41]. The preventive action against spontaneous heat loss can be the installation of an energy barrier for the reverse-phase change from a high-energy phase to a low-energy phase. However, the installation of common energy barrier by using mechanical triggering is limited by the high cost for large-scale applications. Phase changes are influenced by intermolecular interactions, such as van der Waals, dipolar, and hydrogen bonding, which indicate that the phase- transition temperatures and thermal energy densities can be controlled by regulating these key interactions between constituents. Organic photo switches that undergoes reversible isomerization under irradiation of light can be integrated with various materials for smart applications. It is expected that this property can be utilized to alter the physical properties of surrounding molecules through the change in intermolecular interactions.
Figure 4:Water-heating experiments. Showing the temperature changes of 1g of water (blue curve) in contact with 3g of (a), ultraviolet-charged phase-change materials (PCM) composite (35mol% of cis azobenzene dopant (compound 1)) in the dark. (b) Optically triggered composite by the exposure to a blue light emitting diode lamp at 31 °C for 500s to maximize the discharging of cis azobenzene, (c) pristine PCM (i.e., tridecanoic acid), (e) octadecane, and (f) water bath. The inset of (e) shows the setup where each thermocouple is submerged in a heating medium and in a vial containing water. The temperature of water in the vial is at 21-22 °C under ambient condition and quickly increases as the vial is immersed in the heating medium at 40 °C. The temperatures of the heating medium (TPCM) and water (T water) are recorded simultaneously after TPCM reaches 41-42 °C while the heating fluid is stirred. (d) Temperature change of pristine PCM under ambient condition without the process of dipping a water container into the PCM heating bath.
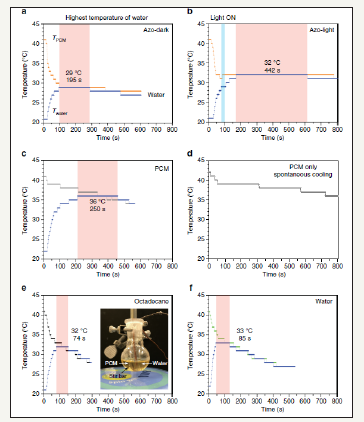
Thus, the doping of azobenzene into conventional organic PCMs can be considered as an approach to change the intermolecular dynamics. These dopants have activation energy barriers for switching between the photo isomers, which provide stability to the phase storing thermal energy and ability for energy release. Thus, high-density energy storage is controllable and scalable in organic composites. Specifically, the azobenzene dopants that change conformation upon illumination can be locked in the liquid phase of PCMs by lowering their crystallization temperature (Tc), retaining the thermal energy storage at cooler temperatures [42]. The release of heat of optically triggered UV-charged PCM composite, which is transferred to water its temperature can be seen in Figure 4.
For example, with n-fatty acids having–COOH groups, the polar interaction and H-bonding between the acid groups can impact the lamellar formation. The azobenzene dopants possess strong π–π interactions among adjacent aromatic cores and van der Waals interactions between alkyl chains. Ester linkers are also expected to contribute to intermolecular interactions.
In the first stage of the thermal storage cycle, the external thermal energy is absorbed by the solid composite in crystalline state. Above the melting point (Tm) of the PCM (42 °C), the composite becomes a mixture of molten PCM and crystalline aggregates of the azobenzene dopant, which has a higher melting point of 73 °C. Then during UV illumination of the slurry, the trans azobenzene dopants isomerizes to cis, which having bent conformation remains well dispersed in the liquid PCM. To keep the temperature of the composite above 42 °C during the UV-charging process, simultaneous heating and UV absorption processes are adopted [43]. The total heat stored by liquid composite comprises the fractional latent heat of the PCM (177Jg-1), that of trans azobenzene dopants (118Jg-1), as well as the fractional isomerization energy of the metastable cis azobenzene (116Jg-1). It is also observed that the liquid state of the composite is conserved through subsequent cooling to a temperature unusual heat storage ability of the composite is achieved by the metastable cis-dopants that does not allow the packing of PCM molecules through steric repulsion and dipolar interactions. Visible-light illumination rapidly isomerizes the dopants back to trans state and allows the PCM composite to crystallize and release the stored latent heat as required, recovering the original state of the composite.
Conclusion
Attempts are being made to utilize suitable PCM composite with carbon-based Nano materials to tap solar energy and store it to mitigate the over dependence on electricity. Carbon based Nano materials such as SWNT, GO, rGO etc. found to be ideal materials which make shape stabilization possible with very low dose incorporation in PCM. Nano carbons are responsible for absorption of photon from sunlight and PCM acts towards storage. Further, the physical blending allows minimum surrender of original enthalpy of PCM and the other important characteristics is the high thermal conductivity of nanocarbons, which makes the system to respond very fast. The search for most suitable material combinations has just started. This is expected to yield rich dividend in near future as more efficient materials will be developed with umpteen combinations.
References
- Abdullahi KL, Delgado SJM, Harrison RM (2013) Emissions and indoor concentrations of particulate matter and its specific chemical components from cooking: A review. Atmospheric Environment 71: 260-294.
- Ying W, Guo F, Li J, Zhang Q, Wu W, et al. (2012) Series of new D-A-π-A organic broadly absorbing sensitizers containing is indigo unit for highly efficient dye-sensitized solar cells. ACS Appl Mater Interfaces 4(8): 4215-4224.
- Hao F, Wang X, Zhou C, Jiao X, Li X, et al. (2012) Efficient light harvesting and charge collection of dye-sensitized solar cells with (001) faceted single crystalline anatase nanoparticles. J Phys Chem C 116(36): 19164- 19172.
- Dai L (2013) Functionalization of graphene for efficient energy conversion and storage. Acc Chem Res 46(1): 31-42.
- Shengqiang X, Samuel CP, Huaxing Z, Wei Y (2010) Recent progress on highly efficient bulk heterojunction polymer solar cells. ACS Symposium Series 1034: 71-80.
- Liu C, Li F, Lai PM, Cheng HM (2010) Advanced materials for energy storage. Adv Mater 22(8): E28-62.
- Granqvist CG (2003) Solar Energy Materials. Adv Mater 15(21): 1789- 1803.
- Han Z, Fina A (2011) Thermal conductivity of carbon nanotubes and their polymer nanocomposites: A review. Prog Polym Sci 36(7): 914- 944.
- Nuraje N, Dang X, Qi J, Allen MA, Lei Y, et al. (2012) Bio templated synthesis of perovskite nanomaterials for solar energy conversion. Adv Mater 24(21): 2885-2889.
- Kam NWS, Connell MO, Wisdom JA, Dai H (2005) Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc Natl Acad Sci USA 102(33): 11600-11605.
- Miyako E, Nagata H, Hirano K, Hirotsu T (2008) Carbon nanotubepolymer composite for light-driven microthermal control. Angew Chem Int Ed 47(19): 3610-3613.
- Eijiro M, Hideya N, Ken H, Kotaro S, Yoji M, et al. (2008) Photoinduced antiviral carbon nanohorns. Nanotechnology 19(7): 075106.
- Bachilo SM, Strano MS, Kittrell C, Hauge RH, Smalley RE, et al. (2002) Structure-assigned optical spectra of single-walled carbon nanotubes. Science 298(5602): 2361-2366.
- Reed BW, Sarikaya M (2001) Electronic properties of carbon nanotubes by transmission electron energy-loss spectroscopy. Phys Rev B 64(19): 5404.
- Reed BW, Sarikaya M, Dalton LR, Bertsch GF (2001) Transmission electron energy-loss spectroscopy study of carbon nanotubes upon high temperature treatment. Appl Phys Lett 78(21): 3358.
- Attal S, Thiruvengadathan R, Regev O (2006) Determination of the concentration of single-walled carbon nanotubes in aqueous dispersions using uv-visible absorption spectroscopy. Anal Chem 78(23): 8098- 8104.
- Sarier N, Onder E (2007) Thermal characteristics of polyurethane foams incorporated with phase change materials. Thermochim Acta 454(2): 90-98.
- Aljaafari AA, Ibrahim SS, El BTA (2011) Thermophysical and electrical characterization of PVC-SWNT nanocomposites. Composites Part A 42(4): 394-399.
- Harish S, Ishikawa K, Einarsson E, Aikawa S, Chiashi S, et al. (2012) Enhanced thermal conductivity of ethylene glycol with single-walled carbon nanotube inclusions. Int J Heat Mass Transfer 55(13-14): 3885- 3890.
- Ismail KAR, Lino FAM (2011) Fins and turbulence promoters for heat transfer enhancement in latent heat storage systems. Exp Therm Fluid Sci 35(6): 1010-1018.
- Agyenim F, Hewitt N, Eames P, Smyth M (2010) A review of materials, heat transfer and phase change problem formulation for latent heat thermal energy storage systems (LHTESS). Renew Sustain Energy Rev 14(2): 615-628.
- McCann JT, Marquez M, Xia Y (2006) Melt coaxial electrospinning: a versatile method for the encapsulation of solid materials and fabrication of phase change nanofibers. Nano Lett 6(12): 2868-2872.
- Chen L, Zou R, Xia W, Liu Z, Shang Y, et al. (2012) Electro and photo driven phase change composites based on wax-infiltrated carbon nanotube sponges. ACS Nano 6(12): 10884-10892.
- Denis PA, Iribarne F (2010) Monolayer and bilayer graphene functionalized with nitrene radicals. J Phys Chem C 115(1): 195-203.
- Akhiani AR, Mehrali M, Latibari ST, Mehrali M, Mahlia TMI, et al. (2015) One-step preparation of form-stable phase change material through self-assembly of fatty acid and graphene. J Phys Chem C 119(40): 22787- 22796.
- Xiong W, Chen Y, Hao M, Zhang L, Mei T, et al. (2015) Facile synthesis of PEG based shape-stabilized phase change materials and their photothermal energy conversion. Appl Therm Eng 91: 630-637.
- Yang J, Qi GQ, Liu Y, Bao RY, Liu ZY, et al. (2016) Hybrid graphene aerogels/phase change material composites: Thermal conductivity, shape-stabilization and light-to-thermal energy storage. Carbon 100: 693-702.
- Wang Y, Tang B, Zhang S (2013) Single-walled carbon nanotube/phase change material composites: sunlight-driven, reversible, form-stable phase transitions for solar thermal energy storage, Adv Funct Mater 23(35): 4354-4360.
- Sun H, Deng J, Qiu LB, Fang X, Peng HS (2015) Recent progress in solar cells based on one-dimensional nanomaterials. Energy & Environ Sci 8(4): 1139-1159.
- Ji HX, Sellan DP, Pettes MT, Kong XH, Ji JY, et al. (2014) Enhanced thermal conductivity of phase change materials with ultrathin-graphite foams for thermal energy storage. Energy Environ Sci 7(3): 1185-1192.
- Kholmanov I, Kim J, Ou E, Ruoff RS, Shi L (2015) Continuous carbon nanotube-ultrathin graphite hybrid foams for increased thermal conductivity and suppressed subcooling in composite phase change materials. ACS Nano 9(12): 11699-11707.
- Qi G, Yang J, Bao R, Xia D, Cao M, et al. (2017) Hierarchical graphene foam-based phase change materials with enhanced thermal conductivity and shape stability for efficient solar-to-thermal energy conversion and storage. Nano Res 10(3): 802-813.
- Kenisarin M, Mahkamov K (2007) Solar energy storage using phase change materials. Renew Sustain Energy Rev 11(9): 1913-1965.
- Muthusivagami RM, Velraj R, Sethumadhavan R, (2010) Solar cookers with and without thermal storage-A review, Renewable and Sustainable Energy Rev. Elsevier 14(2): 691-701.
- Merlin K, Delaunay D, Soto J, Traonvouez L (2016) Heat transfer enhancement in latent heat thermal storage systems: Comparative study of different solutions and thermal contact investigation between the exchanger and the PCM. Appl Energy 166: 107-116.
- Santhi Rekha SM, Sukchai S (2018) Design of phase change material based domestic solar cooking system for both indoor and outdoor cooking applications. J Sol Energy Eng 140(4): 041010-041018.
- Mazer JA (1997) Solar cells: An introduction to crystalline photovoltaic technology. Kluwer Academic Publishers, p. 216.
- Radziems KAE, Klugmann E (2002) Thermally affected parameters of the current-voltage characteristics of silicon photocell. Energy Convers Manag 43(14): 1889-1900.
- Jiang JA, Wang JC, Kuo KC, Su YL, Shieh JC, et al. (2012) Analysis of the junction temperature and thermal characteristics of photovoltaic modules under various operation conditions. Energy 44(1): 292-301.
- Gur I, Sawyer IK, Prasher R (2012) Searching for a better thermal battery. Science 335(6075): 1454-1455.
- Han GGD, Li H, Grossman JC (2017) Optically-controlled long-term storage and release of thermal energy in phase-change materials 8(1): 1446.
- Gbabode G, Négrier P, Mondieig D, Calvo EM, Calvet T, et al. (2007) Structures of the high-temperature solid phases of the odd numbered fatty acids from tridecanoic acid to tricosanoic acid. Chem Eur J 13(11): 3150-3159.
- Ueki T, Nakamura Y, Yamaguchi A, Niitsuma K, Lodge TP, et al. (2011) UCST phase transition of azobenzene-containing random copolymer in an ionic liquid. Macromolecules 44(17): 6908-6914.
© 2018 Asit Baran Samui. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






