- Submissions

Full Text
Perceptions in Reproductive Medicine
Successful Transplacental Antiarrhythmic Treatment of Supraventricular Tachycardia in a Fetus with Ebstein Anomaly
Márta Katona1*, Tamas Bitó2, Katalin Rácz1 and Hajnalka Orvos2
1Department of Pediatrics and Pedriatic Health Center, University of Szeged, Hungary
2Department of Obstetrics and Gynecology, University of Szeged, Hungary
*Corresponding author:Marta Katona, Department of Pediatrics and Pedriatic Health Center, Albert Szent-Györgyi Health Center, University of Szeged, Hungary Korányi fasor 14-15, 6720 Szeged e-mail: katona.marta@med.uszeged.hu
Submission: June 04, 2025;Published: July 16, 2025

ISSN: 2640-9666Volume6 Issue 3
Abstract
Our case presents a transplacental combined antiarrhythmic treatment lasting two months in a fetus with severe congenital heart defect. Fetal echocardiography revealed fetal supraventricular tachycardia and Ebstein anomaly. Digoxin monotherapy was not efficient alone, the combination with Verapamil was successful; the arrhythmia was converted to sinus rhythm after 15 days, the development of fetal hydrops and premature delivery were prevented. The importance of decision making of a multidisciplinary team and the very strict monitorization of both the mother and fetus is emphasized in order to avoid side effects of antiarrhythmic treatment After delivery a term baby was born, who underwent successful palliative cardiac surgery in the newbon period. The ECG showed Wolff-Parkinson-White syndrome, which did not require antiarrhythmic therapy after birth. A personalized transplacental pharmacologic treatment of a life-threatening arrhythmia could improve the outcome of even a very severe fetal cardiac disease.
Abbreviations: AIUM: American Institute of Ultrasound in Medicine; AV: Atrio-Ventricular; CTG: Cardiotocography; ECG: Electrocardiography; FHR: Fetal Heart Rate; GW: Gestational Week; CHD: Congenital Heart Defect; SVT: Supraventricular Tachycardia
Introduction
Fetal arrhythmia relatively frequently occurs during gestation with an incidence of about 1-2% of all pregnancies. The most frequent fetal arryhtmias are sinus tachycardia, premature atrial and ventricular contractions and ectopic beats. In the majority of cases fetal arrhythmias are benign and do not require treatment. However, 10% of these arrhythmias (fetal supraventricular tachycardia, complete atrio-ventricular block, ventricular tachycardia) may lead to life- threatening complications including fetal hydrops, fetal death or premature termination of the pregnancy. Fetal tachycardia can be treated prenatally with different antiarrhythmic drugs, the first-line choice being Digoxin, or more recently Flecainide [1].
Case Report
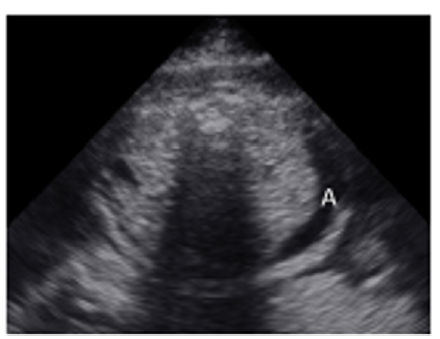
A 35-year-old pregnant woman being in the 29th Gestational Week (GW) was admitted to the Department of Obstetrics and Gynecology of University of Szeged, due to fetal tachycardia with a Fetal Heart Rate (FHR) of 250/min. The mother suffered from hypertension before the pregnancy and underwent calcium channel blocker therapy (Verapamil), which was replaced with methyldopa (Dopegyt) during the pregnancy. Nuchal translucency and biomarkers measured in the first trimester were normal. There was no positive family history for Congenital Heart Defect (CHD). A fetal echocardiography was performed, according to the AIUM guidelines [1]. The FHR was between 240-260/min, the Atrio-Ventricular (AV) conduction was 1:1, a Supraventricular Tachycardia (SVT) was diagnosed (Figure 1). Fetal echocardiography revealed an abnormal four-chamber view, a very large right atrium and a very small right ventricle, the tricuspid valve was abnormal, attached deeply in the right ventricle and the apical displacement of the valve led to the atrialization of the right ventricle and a holosystolic tricuspid regurgitation could be detected by Doppler. The pulmonary artery was very narrow, the diameter of the aorta was twice as big as the diameter of the pulmonary artery, therefore a duct-dependent CHD with decreased pulmonary circulation was suspected. The abnormal anatomy of the fetal heart was characteristic for Ebstein anomaly (Figure 2), which is a complex cyanotic CHD. Pericardial or pleural effusion was not observed, but some ascites was already seen in the abdominal cavity (Figure 3), indicative of incipient fetal heart failure.
Figure 1:Supraventricular tachycardia in a 29 GW old fetus. FHR. 250/min.
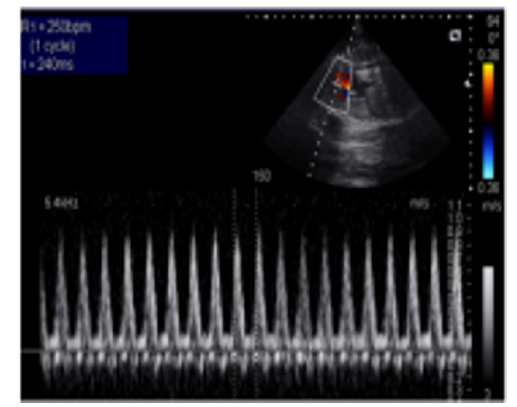
Figure 2:Abnormal four-chamber view of the 29 GW old fetus; large right atrium, very small right ventricle, abnormal apical displacement of the tricuspid valve: Ebstein anomaly (RA=right atrium, RV=right ventricle, LA=left atrium, LV=left ventricle).
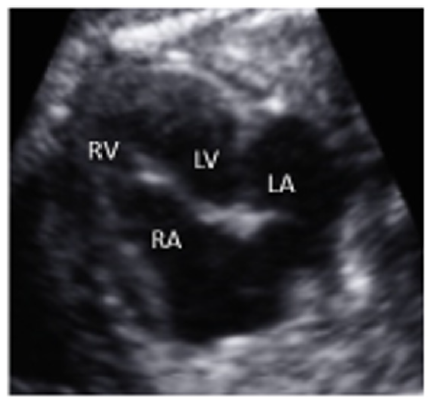
Figure 3:Transversal abdominal view of the 29 GW old fetus: abdominal fluid, ascites can be seen in the fetal abdomonal cavity (A=ascites).
Multidisciplinary team consultation
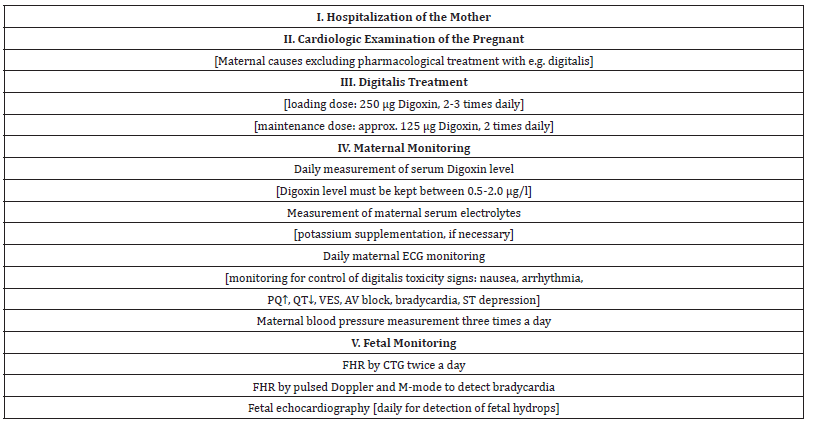
A multidisciplinary team consultation was organized among the obstetrician, the perinatal cardiologist, the neonatologist, the cardiologist, the pediatric cardiac surgeon and the geneticist. The common decision was to start transplacental antiarrhythmic therapy to convert the fetal SVT into sinus rhythm, to prevent the development of fetal hydrops and to avoid premature delivery or premature termination of the pregnancy (Table 1). The risk of a cardiac surgery in a premature baby is much higher, than the risk of prenatal antiarrhythmic therapy. The treatment should be personalized; an individual therapy is necessary regarding the mother’s health satus and the fetal condition. A very strict monitoring of the cardiac funtion of both the mother and the fetus is necessary, in order to prevent proarrhythmic side effects of the antiarrhythmic drugs. Specialists trained in perinatal cardiology and in maternal-fetal medicine should be involved in the treatment. According to the guidelines and our previous experiences, we decided to start transplacental Digoxin as first-line therapy in the treatment of fetal SVT [2-8].
Table 1:Steps in planning of transplacental antiarrhythmic treatment.
Digitalis treatment was started as monotherpy; oral Digoxin (2 x 250μg) was given for several days, but unfortunately it was not successful, since FHR remained above 240/min, in spite that the loading serum level of Digoxin was reached (1,2μg/l). Combined antiarrhythmic therapy was necessary after 8 days, therefore oral Verapamil (40 mg twice a day) was introduced as an additional second medication [2,4,6,9]. The reason, that we chose Verapamil as a second drug, was maternal hypertension and danger of premature delivery, as Verapamil also has tocolytic effect. After 11 days, FHR decreased to 140/min and SVT was converted to sinus rhythm. Unfortunately, the FHR increased very soon again, recurrence of SVT (260/min) was detected 2 days later, so we had to further increase the dose of Verapamil (first 2 x 60mg, later 2 x 80 mg). Fortunately, fetal hydrops did not develop during the observation period. After 15 days fetal sinus rhythm returned again and the combined antiarrhythmic therapy was continued. When we tried to reduce the dose of any of the two medications, FHR increased again up to 250/min. At the age of 30 GW steroid injection (Oradexon) was given to the mother 2 times to promote surfactant production in the lung of the fetus, in order to prevent the development of severe respiratory distress syndrome in case of a premature delivery. Maternal ECG was regularly controlled; no signs of digitalis toxicity was noted, she had normal PQ, QRS, QT and QTc intervals and normal repolarisation. The serum Digoxin level was within the therapeutic range. Maternal blood pressure was normal, neither significant increase, nor decrease could be measured. Fetal echocardiography (2-dimensional, M-mode, pulsed Doppler and color Doppler) was performed every day, until permanent sinus rhythm was not achieved. The FHR, the AV conduction, the size of the cardiac chambers, ejection fraction, contractility of the heart, the degree of tricuspid regurgitation, presence of pericardial or pleural effusion were examined every day.
On the 38th GW an elective cesarean section was performed. A slightly small for date newborn baby with a birth weight of 2800g was delivered with a markedly decreased oxygen saturation (88%), and a normal heart rate of 145/min. Since the newborn had a stable sinus rhythm, the antiarrhythmic treatment was discontinued. ECG examination of the newborn showed short PQ interval and a delta wave, signs of Wolff-Parkinson-White syndrome. Dopplerechocardiography supported the findings of the fetal examinaton and the diagnosis of Ebstein anomaly with duct-dependent pulmonary circulation was confirmed. Prostaglandin E1 infusion was started and at the age of 8 days a palliative cardiac surgery was performed (a Blalock- Taussig shunt to improve the pulmonary blood flow). Follow-up examinations revealed no evidence for neurodevelopmental or cognitive disability; at the age of 10 years the patient visits regular school and he is waiting for the total correction of Ebstein anomaly.
Summary
Pharmacologic treatment of a life-threatening arrhythmia in the fetus is always challenging and requires great precautions [2]. A decision by a multidisciplinary team carries great responsibility. In case of fetal arrhythmia a personalized treatment of transplacental antiarrhythmic therapy is very important [9]. Safety of both the pregnant and the fetus is mandatory to prevent side effects of the medication and avoiding maternal or fetal deterioration [5]. Fetal echocardiography is an excellent tool not only to detect structural abnormality of the fetal heart but also to assess fetal cardiac function [3]. Until very recently Digoxin, as a class V antiarrhythmic drug was regarded as the first-line treatment of fetal SVT. More recent evidence suggests Flecainide, a class Ic antiarrhythmic drug as first-line treatment of SVT [2,9]. However, Sotalol, a class III and II antiarrhythmic drug was also reported to be used as a firstline therapy. In case of fetal hydrops Flecainide proved to be more effective. Amiodarone, a class III antiarrhythmic drug is a secondline therapy in the treatment of fetal SVT, as it could lead to fetal hypothyreosis [2]. In case of drug-refractory fetal tachycardia a combination of antiarrhythmic drugs should be considered. At present the leading cardiology centers suggest the combination of Digoxin and Flecainide to replace the Digoxin and Verapamil combination, which was the standard treatment of SVT for many years [2]. Strict inpatient monitoring of maternal and fetal cardiac status should be carried out [5]. Measurement of maternal serum antiarrhythmic drug level is mandatory to achieve therapeutic effect and to avoid proarrhythmic side effects [9]. Hospitalization of the pregnant, regular maternal ECG, blood pressure measurements, CTG and Fetal echocardiography are necessary. Prevention of the development of fetal hydrops can avoid premature delivery. Timing and mode of the delivery depends on the fetal well-being, the underlying cardiac disease and the hemodynamic condition of the fetus, which can be assessed by the obstetrician and the perinatal cardiologist. The aim of our case presentation was to present the different aspects of a prenatal combined antiarrhythmic treatment and to show that with careful planning by a multidisciplinary team, the outcome and prognosis of even a very severe fetal cardiac disease can be successful.
Conclusion
The pharmacologic treatment of fetal SVT requires a careful decision of a multidisciplinary team, experienced in perinatal cardiology. First-line therapy is either Digoxin or recently Flecainide, if monotherapy is unsuccessful, combined antiarrhythmic therapy (Digoxin and Flecainide) is necessary to prevent fetal hydrops, fetal death or premature delivery. Strict monitoring of the maternal and fetal cardiac function is mandatory in oder to avoid side effects of the antiarrhythmic drugs. Fetal echocardiography is an excellent tool in establishing the diagnosis of the fetal cardiac disease and to assess the effect of the antiarrhythmic therapy as well.
References
- American Institute of Ultrasound in Medicine AIUM (2013) Practice Guideline for the performance of fetal echocardiography Practice Guideline, Ultrasound Med 32(6): 1067-1082.
- Batra AS, Silka MJ, Borquez A, Cuneo B, Dechert B, et al. (2024) Pharmacological management of cardiac arrhythmias in the fetal and neonatal periods: A scientific statement from the American heart association: Endorsed by the Pediatric & Congenital Electrophysiology Society (PACES) Circulation 149(10): e937-e952.
- Crispi F, Valenzuela-Alcaraz B, Cruz-Lemini M, Gratacós E (2013) Ultrasound assessment of fetal cardiac function. AJUM 16(4): 158-167.
- Engelhardt W, Grabitz RG, Funk A, Bernuth GV (1993) Intrauterine therapy of fetal supraventricular tachycardia with digoxin and verapamil. Z Geburtshilfe Perinatol 197(2): 99-103.
- Malhamé I, Gandhi C, arabulsi G, Esposito M, Lombardi K, et al. (2018) Maternal monitoring and safety considerations during antiarrhythmic treatment of fetal supraventricular tachycardia. Obstet Med 12(2): 66-75.
- Muncz JL, Lewis AL, Song J, Ramsey PS (2022) Fetal intervention for refractory supraventricular tachycardia complicated by hydrops fetalis. Case Rep Obstet Gynecol 2022: 5148250.
- Purkayastha S, Weinreich M, Fontes JD, Lau JF, Wolfe DS,et al. (2022) Fetal supraventricular tachycardia: What the adult cardiologist needs to know. Cardiol Rev 50(1): 31-37.
- Strasburger J, Eckstei G, Butler M, Noffke P, Wacker-Gussmann A (2022) Fetal arrythmia diagnosis and pharmacologic management. J Clin Pharmacol 62(Suppl 1): S53-S66.
- Palmen R, Sandritter T, Malloy-Walton L, Follansbee C, Wagner JB (2023) Case report: Use of therapeutic drug monitoring and pharmacogenetic testing as opportunities to individualize care in case of flecainide toxicity after fetal supraventricular tachycardia. Front Pediatr 11: 1168619.
© 2025 Márta Katona. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






