- Submissions

Full Text
Perceptions in Reproductive Medicine
Development of Systematic and Active Technovigilance for Male Condoms Sold in Digital Markets
Janete Duarte1*, Adriana Sant’Ana da Silva1, Jarbas Emilio dos Santos1, Renata Gracie2 and Jislaine de Fatima Guilhermino3
1National Institute for Quality Control in Health (INCQS)/Fiocruz, Brazil
2Institute of Scientific and Technological Communication and Information, (ICICT)/Fiocruz,Brazil
3Fiocruz Mato Grosso do Sul, Brazil
*Corresponding author:Janete Duarte, Oswaldo Cruz Foundation, National Institute for Quality Control in Health (INCQS)/Fiocruz, Brazil
Submission: November 04, 2024;Published: November 19, 2024

ISSN: 2640-9666Volume6 Issue2
Abstract
Background: Products such as male condoms, which are made from natural rubber, must undergo
rigorous quality control before being made available to the public. In Brazil, condoms, both domestic
and imported, are compulsorily certified and their production is governed by strict criteria, which
cover everything from the quality of the latex to the specifications for primary packaging, packaging for
consumption and transportation. However, although the certification process assesses the production and
the product in detail at the end of manufacturing, it does not address the issue of after-sales service in the
various establishments, issues typical of Sanitary Surveillance actions.
Methods: Three batches of male condoms from different brands, purchased from digital marketplaces,
were evaluated. The samples were anonymously identified as (Sample A), (Sample B), and (Sample C).
A total of 288 units were purchased per batch. Based on the number of units purchased per batch, the
inflation test was performed, which assesses resistance by determining the volumetric capacity and burst
pressure. For the inflation test, we tested 200 units per brand, according to the criteria established in the
Resolution of the National Sanitary Surveillance Agency No. RDC 554/2021. In one of the samples, it was
necessary to perform the aging test, sample stability, before the insuflation test (Sample B).
Results and Conclusion: All brands met the criteria established in the Resolution of the National Sanitary
Surveillance Agency No. RDC 554/2021 for the inflation test, which allows up to seven non-compliant
units; however, non-conformities were found in all of them. We conclude that, in addition to certification,
there is a need to monitor this product in view of the health risk observed, and a systematic and active
techno vigilance system can provide a new dimension to the National Sanitary Surveillance System in
monitoring this product in digital markets.
Introduction
Background of the study
Natural rubber has excellent properties such as tensile, abrasion and tear resistance and, combined with high elasticity, the large number of un saturations present in the polymer chain, even after vulcanization of the material, causes aging due to the action of temperature, oxygen, ozone and radiation, with gradual loss of these properties [1]. The combination of excellent mechanical properties combined with biocompatibility makes it a material widely used in the manufacture of health products. These products are processed from poly(cis-isoprene) latexes extracted from rubber trees. However, the large number of unsaturations present in the polymer chain, even after vulcanization, results in low resistance of this material to aging, caused mainly by the combination of factors such as temperature, oxygen, ozone and radiation. The presence of proteins in latex, with allergenic potential, can promote physiological reactions in some users [2].
The process used worldwide so that latex can be used commercially is the conventional vulcanization of rubber, which consists of the crosslinking of poly-1,4-cis(isoprene) promoted by heat, in the presence of sulfur and additives, which allow for major transformations in the properties of the rubber, such as reduced solubility in organic solvents, increased mechanical resistance, greater resistance to acids and alkalis, among others [3]. The conversion of natural rubber latex into products can occur through several processes, which are characteristic of latex technology. These processes consume little energy in mixing, molding and vulcanization, but a lot of energy in drying. This fact restricts latex technology to the manufacture of products with thicknesses of less than 3mm.
In order to obtain rubber products with properties that can find industrial applications, it is necessary to transform the plastic properties of rubber into elastic ones. It is necessary to have a broad knowledge of the properties and end use of the product in order to properly formulate the latex compound and minimize the number of additives used, especially when the application involves contact with living organisms [4]. In the vulcanization of latex for the production of products such as male condoms, an ideal balance is sought between elasticity and resistance, as both are important qualities for the performance of the product [5]. Products such as male condoms, which are made from natural rubber, must undergo rigorous quality control before being made available to the public. In Brazil, condoms, both domestic and imported, are compulsorily certified and their production is governed by strict criteria, which cover everything from the quality of the latex to the specifications for primary packaging, packaging for consumption and transportation. However, although the certification process assesses the production and the product in detail at the end of manufacturing, it does not address the issue of after-sales service in the various establishments, issues typical of Sanitary Surveillance actions.
As medical devices, mechanical contraceptives require regulation in terms of market approval, efficacy, quality and fitness for purpose. ISO 4074, Natural latex rubber condoms - Requirements and test methods, establishes all the criteria and methods for evaluating male condoms [6]. Monitoring this product is difficult because spontaneous demand is almost non-existent, as it is a product associated with sexual behaviour and stigmatized, mainly due to conditions of use, and social issues. However, it is under the aegis of legislation that has as its premise the need to use the Attribute Sampling Plan, where the number of units to be analysed is very high, making it difficult to acquire units of the same batch on the market in order to meet the criteria established by legislation. In addition to these difficulties, the product also escapes the focus of monitoring because it has found a large sales space in digital commerce.
Sanitary Surveillance, as an integral part of the Unified Health System (SUS), is a priority competence due to its preventive and corrective nature, and should act to improve the population’s quality of life. Its actions are aimed at preventing, reducing and eliminating risks to the population’s health, arising from the environment, production, circulation of goods and provision of services. As a public policy, it is no different from others and should seek improvement through effective, planned action, avoiding overlapping actions or lack thereof, with monitoring the quality of goods and products intended for health post-marketing being one of its fundamental aspects [7].
The only practical method for assessing the quality of condoms is to characterize a representative sample of a batch or series of batches. This is because natural rubber condoms are articles produced in large production batches, ranging from 150,000 to 500,000 units. Therefore, individual variations in product quality inevitably occur. In this context, it is foreseeable that a small proportion of condoms in a production batch may not meet the requirements established in the product quality standards. In addition, the tests used to evaluate the product are destructive tests [8-10].
The presence of low-quality condoms on the market represents a serious challenge in the fight against STIs. The lack of quality of this product affects the popular perception of the value of the condom, which, in turn, can have a significant impact on public health. Therefore, they can harm not only the health of users, but also the reputation of agencies or bodies that provide them. The quality of condoms available in Brazil is under the jurisdiction of the National Sanitary Surveillance Agency (ANVISA) of the Brazilian Ministry of Health. Resolution No. 554/2021 of the National Sanitary Surveillance Agency, based on ISO 4074 standards, has the Sampling Plan by Attributes, ISO 2859-1 as sampling criteria, establishes the mandatory certification of natural rubber latex male condoms, both national and imported, before their sale or free distribution to consumers. The table below presents the criteria established by ISO standards and Resolution of the National Sanitary Surveillance Agency - ANVISA No. 554/2021 [11] for the quality control of male condoms. The development of Systematic and Active Technovigilance in digital markets expands Sanitary Surveillance actions for male condoms, imprints a process of innovation in the National Sanitary Surveillance System, as this System will be anchored in laboratory analyses in a Public Health laboratory and will be georeferenced throughout the sample acquisition process.
Methods
Initially, we researched digital markets as a pilot study to establish how to acquire samples from the same batch. We were able to acquire three batches of 288 units each. The insuflation test has historically been used as an indicator of condom resistance, measuring the pressure and burst volume. The main advantage of this test is the almost complete evaluation of the condom, such as, for example, sensitivity to localized flaws in the film and the possibility of correlating poor performance in the test with degradation and aging, as well as the rate of rupture in use. For condom samples up to one year old, the standard establishes that the test determines the stability of the sample after aging before the volumetric capacity and burst pressure test. As shown in Table 1, and with the number of units per batch acquired, this is the best test to show the profile of condoms sold in these markets.
Table 1:Criteria established by Resolution of the National Sanitary Surveillance Agency (ANVISA) No. 554/2021 554/2021 for physical tests for male condoms.
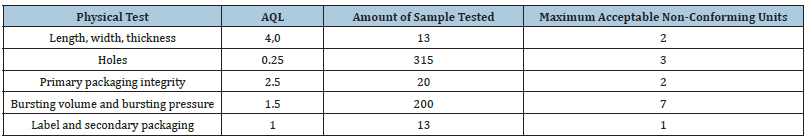
AQL - Acceptable Quality Level.
The three batches of different brands of male condoms, anonymized by letter marking, purchased on digital markets, contained 288 units each and renamed as: Sample A, Sample B, Sample C. The test to determine the volumetric capacity and burst pressure was carried out on the three batches purchased, and in one of them (Sample B) it was necessary to first determine the stability of the sample after aging. In determining the volumetric capacity and burst pressure, inflation test, in all regulations, the burst volume requirement is linked to the nominal width of the condom, measured at 75±5mm from the closed end, excluding the reservoir tip. The burst volume and burst pressure were measured in accordance with the standards established by Resolution No. RDC 554/2021 of the National Sanitary Surveillance Agency.
Determination of volumetric capacity and burst pressure - insuflation test
For this analysis, we used the ISO 2859 single sampling plan [9], with a normal inspection regime, at inspection level I (Acceptable Quality Level (AQL)=1.5, less than 1.5% of units defective). The number of units assessed was 200 per lot, and the acceptance criteria reject the lot if at least eight nonconforming units were identified. During the insufation test, the condom is inflated like a balloon, stretching the latex film until its rupture, thereby indicating its maximum resistance. The inflation system is accompanied by software that logs the pressure and volume at bursting. The compressed air that supplies the system is generated by a dry, oilfree air compressor. The flow of compressed air was set at 24-30dm3 min-1, as defined in the standards. For each condom, the bursting pressure (1kPa=N force applied uniformly over an area of 1m2) and bursting volume (in dm3). The equipment used the four-head automated inflation system (Enersol™, Sydney, Australia) logged via the Inflation version 3.0.4.0 software by ENERSOL Serving the Global Medical Device Industry [10].
Determination of stability after aging
The stability test, which consists of aging, as defined in the legislation for condoms manufactured less than one year ago, was performed on sample B. The sample was conditioned in an oven at (70±2) ºC for (168±2)h. It was removed from the oven and kept in the package at (25º±5º)C for a period of 12 hours. The packages were opened and the condoms examined for signs of deterioration, signs of stickiness, friability among other things that could affect the quality of the product before the volumetric capacity and burst pressure tests.
Results
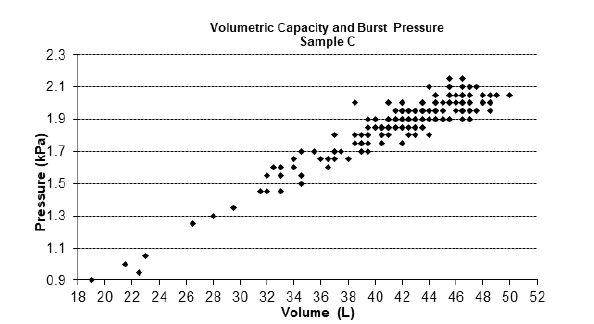
For the physical tests, Table 1 shows the NQA, quantity of sample tested and the criteria established in accordance with RDC 554/2021. Figures 1-3 represent the graphs of the insuflation tests performed on samples A, B and C respectively. The variability for burst pressure and volume shows that the units are not uniform. However, the distribution extends to the left for both pressure and volume, characterizing a non-normal distribution contaminated by outliers, but within the compliance area. The graphs show that all samples analysed presented non-conformities in volume and pressure or in one or the other of these criteria. In Sample A, we found one non-conformity in the volume criterion, in Sample B, two non-conformities were found in both criteria, volume and pressure, and in Sample C, six non-conformities were found, two for the pressure criterion and four for the volume criterion.
Figure 1:
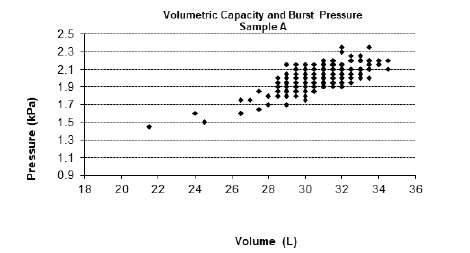
Figure 2:
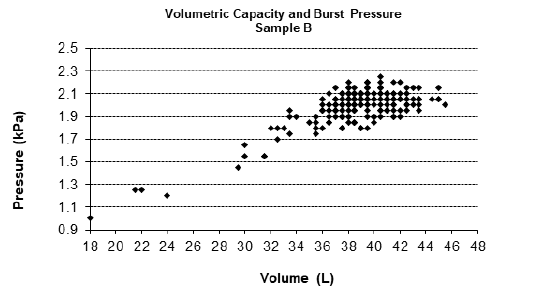
Figure 3:
The standards count “defective sample” and not the “nonconforming” parameter, therefore, the total number of defective samples per batch is not the sum of the number of individual nonconformities by parameter. In this case, in the test to determine the volumetric capacity of burst pressure, the parameters, pressure and volume, should be considered individual parameters when monitored by the Health Surveillance, based on the assessment of the health risk. In the test to determine the volumetric capacity and burst pressure, the number of non-conformities in the burst volume is generally greater than the number of non-conformities in the burst pressure, since when the condoms are non-conforming in the pressure, they are also non-conforming in the volume.
The results observed indicate the need for Sanitary Surveillance action for male condoms in digital markets. With the number of units, we were able to acquire, it was only possible to determine the volumetric capacity and burst pressure. However, to perform the other tests based on what was shown in the results found is very important. The development of a systematic and active technosurveillance for male condoms in digital markets provides a new approach by combining a set of data, asserting itself through the use of physical, microbiological and cytotoxic analytical tests, made available by the Public Health laboratory, combining a technological information tool for mapping and traceability through the study of spatial analysis of geoprocessing, indicating a path that will make it possible to direct Sanitary Surveillance actions, allowing the National Sanitary Surveillance System to respond appropriately to the notifications received.
References
- Cornish K (2001) Similarities and differences in rubber biochemistry among plant. Phytochemistry 57(7): 1123-1134.
- Reich L, Stiva SS (1971) Elements of polymer degradation. McGraw-Hill, United States.
- Gazeley KF, Gorton ADT, Pendle TD (1990) Natural rubber science and technology, Roberts A. D. Edition, United Kingdom.
- (1993) Basic course in elastomer technology.
- Coran AY (1994) Science and technology of rubber, Academic Press, United States.
- ISO (2002) 4074 Natural latex rubber condoms: requirements and test methods, Switzerland.
- Lucchesi G (1992) Health Surveillance: the missing link. Disclosure Health Debate (7): 48-52
- Bó MC (2003) Stability study accelerated and real time ageing 1. In: 20th Meeting ISO TC157/ WG 13, United States.
- ISO (1999) 2859-1 Sampling procedures for inspection by attributes, Switzerland.
- Enersol Consulting Engineers (2023). Software EInflation, USA.
- ANVISA (2021) Establishes the minimum requirements to be that must comply with natural rubber latex male condoms.
© 2024 Janete Duarte. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






