- Submissions

Full Text
Progress in Petrochemical Science
Evaluation of the Corrosion Resistance of the Hooks Used in the Glass Fibre-Reinforced Concrete (GFRC) Industry
Husnu Gerengi1, Muhammed Maraşlı2, Marziya Rizvi3 and Kader Coskun4
1Faculty of Engineering, Department of Mechanical Engineering, Corrosion Research Laboratory, Duzce University, Turkey
2Fibrobeton Company R&D Center, Turkey
3Department of Chemistry, Babasaheb Bhimrao Ambedkar University, India
4Research and Development Center, Standard Profile Corporation, Turkey
*Corresponding author:Marziya Rizvi, Department of Chemistry, Babasaheb Bhimrao Ambedkar University, Lucknow, UP, India
Submission: April 25, 2025;Published: June 03, 2025

ISSN 2637-8035Volume7 Issue 2
Abstract
The purpose of this study is to evaluate the corrosion resistance of the most commonly used steel pad hooks used in the Glass Fibre Reinforced Concrete (GFRC) industry. Stainless Steel (316L) (SS) uncoated (CS) and Centrifugal Hot Dip Galvanised (SHDG) St37 steel hooks have been subjected to salt spray corrosion tests in a 5% NaCl solution for 10 days to simulate harsh industrial conditions in accordance with the ASTM B117 salt spray method. Surface changes were examined at intervals using Scanning Electron Microscopy (SEM) and Energy-Dispersive X-Ray Spectroscopy (EDX) to assess corrosion product formation and morphological alterations. The results demonstrated superior corrosion resistance in SHDG hooks compared to SS hooks, with the SHDG samples showing minimal surface rust and the formation of protective zinc oxide and hydroxide layers. The study concludes that SHDG provides significant corrosion protection for steel components in saline environments, offering an economical and effective solution for enhancing the durability of GFRC structures.
Keywords:Steel hooks; 316 L; St37; Centrifuged hop dip galvanization; ASTM B117
Introduction
Corrosion control and damage repair have become multibillion-dollar problem worldwide. Steel is the most common and versatile metal used in industry. The only problem with it is that it can easily deteriorate and corrode. Austenitic stainless steel is widely used in the petrochemical, hydrometallurgical, nuclear and other industries due to its diverse mechanical properties and corrosion resistance. As a typical austenitic stainless steel, 316 L stainless steel is commonly used for oil pipelines and storage tanks because of its high corrosion resistance to media such as CO2. However, 316 L stainless steel is pitted in an environment with a high concentration of Cl-, posing a potential risk to manufactured equipment [1]. A more commonly used industrial steel is St37 carbon steel. However, it is easily corroded in saline environments, and uniform corrosion is usually observed [2]. Steel hooks are often used with various building materials for anchoring and fixing purposes [3,4]. The steel hooks used while constructing with GFRC are depicted in Figure 1. Pad hooks enable safe and accurate installation of particularly large and heavy GFRC panels. The corrosion protection of these fasteners is critical to both the safety of the installation and the long-term durability of the structure (Figure 2). The use of corrosion resistant materials or the application of appropriate protective coatings helps to minimise these risks [5,6] Unprotected metals that are not corrosion resistant can very quickly undergo some form of deterioration due to inevitable contact with atmospheric elements [7]. The most effective and economically viable method is to galvanize these hooks [8].
Figure 1:Typical steel pad hook used in GFRC construction.

Figure 2:The use of pad hooks in assembly.

Galvanizing requires less maintenance and therefore has lower long-term costs than many other commonly specified protective coatings for steel. The life expectancy of galvanized coatings on typical structural members is 50 years in most rural environments and 20-25 years, even in severe urban and coastal environments. Typically, the galvanizing process is not dependent on weather conditions. Galvanized coatings corrode preferentially to steel, providing cathodic or sacrificial protection to small areas of steel exposed by corrosion. Unlike organic coatings, small damaged areas of galvanized steel need not be affected. Even recesses, sharp corners and inaccessible areas are not easily corroded. No coating applied to a structure or fabrication after completion can provide the same protection. Galvanized coatings can be easily assessed by eye, or simple non-destructive thickness testing methods can be used [9,10]. Galvanization is simply the application of zinc. Zinc has been widely used in the surface treatment of various steel structures to improve their service life because of its good corrosion resistance [11,12]. During galvanization, zinc forms galvanized steel after solidifying on the surface of the steel. The outer layer of galvanized steel is the η-layer (100% Zn), followed by the inner alloy layers: the ζ-layer (94% Zn ∼6% Fe), δ-layer (90% Zn ∼10% Fe) and γ-layer (75% Zn ∼25% Fe) [13-15]. The zinc layer protects the matrix by sacrificing itself and forming corrosion products [16]. In particular, corrosion studies in the scratched area of the zinc layer indicate that these two mechanisms, i.e., pure sacrificial protection and pure barrier protection, can occur simultaneously. Among them, sacrificial protection is dominant, and barrier protection is dominant but occurs before the appearance of iron corrosion products [17-19].
In previous works, researchers investigated the corrosion behaviour of electrogalvanized hooks embedded in GFRCs [20]. Sandblasted electrogalvanized hooks coated with zinc-rich coatings exhibited high corrosion resistance properties after the salt spray test, and almost no corrosion was observed [9]. Zinc coatings are applied to steel surfaces via hot dip galvanizing, electroplating, sherardizing, mechanical plating, painting with zinc-rich coatings and zinc spraying (metallizing). Among these methods, the hot dip galvanizing process is by far the most widely used. Small items such as anchoring hooks can be dipped into the molten zinc in a container that is spun or after the molten zinc is removed. This helps to remove excess zinc from threads and edges and provides a smooth, albeit thinner and more wear-resistant, coating. During high-temperature/centrifugal zinc plating, the hooks undergo a multistage pretreatment process. Excess zinc is automatically removed during the centrifugation process to ensure a smooth surface and perfect fit. The anchor elements and pad hooks are hot-dip galvanized, typically to a thickness of 50-100μm, to provide cathodic and barrier corrosion protection [21]. Galvanization involves immersing a cleaned steel hook in a bath of molten zinc at approximately 470 °C, resulting in a metallurgical reaction between the steel and zinc. The result of galvanizing is a hard, wear-resistant and anti-abrasive zinc coating that can withstand mechanical stress. However, conventional zinc plating techniques can pose challenges when small parts are plated. However, centrifugal hot-dip galvanizing, also known as spin galvanizing, is a unique technique that allows small steel parts to be galvanized with a durable zinc coating [22]. Despite all the benefits of SHDG, it is a layered finished product rather than a fully alloyed product such as 316 L stainless steel. The present study investigated the corrosion behaviour of SHDG steel in simulated industrial atmospheric environments via salt spray accelerated corrosion tests. The tests were designed to compare the corrosion resistance properties of 316 L Stainless Steel (SS) and SHDG St37 carbon steel and S37 hooks in a salt chamber with a 5% (w/v) NaCl solution at 50% humidity for 10 days. Corrosion evaluation was performed according to the ASTM B117 salt spray method, with visual inspections and analyses by Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDAX).
Materials and Experimental Procedures
The chemical composition of the steel in hooks as a percentage of weight is as follows: for CS, it was Carbon (C) at 0.17, Manganese (Mn) at 1.40, Phosphorus (P) at 0.05, Silicon (Si) at 0.30, Sulfur (S) at 0.05, and Iron (Fe) at 98.03. The elemental composition of the SS samples was 0.03% carbon, 1.00% silicon, 2.00% manganese, 0.045% phosphorus, 0.015% sulfur, 16.85% chromium, 11.90% nickel, 0.010% molybdenum, 2.00% iron, and the remainder iron. In the galvanizing facility, uniform standard steel hooks (CSs) were collected in a round basket with numerous drainage vents. The CS hooks were subjected to several preliminary cleaning steps, namely, degreasing, pickling and fluxing. Degreasing removes dirt, oil and organic residues. An acid pickling bath removes the mill scale and iron oxide. Fluxing removes any remaining oxide layers and forms a barrier layer to prevent further oxidation of the cleaned surface. The cleaning process is critical, as zinc does not react with the oxidized steel surface. The CS hooks are then dipped into the molten zinc. After the zinc bath, the basket is rapidly rotated to centrifuge off the excess zinc. The small parts are then quenched in a water bath to prevent them from sticking together. The galvanized steel hooks (SHDGs) are cleaned, weighed, and carefully inspected. Calibrated instrumentation ensures that quality standards are met and that the coating thickness, appearance, and compliance with ASTM specifications (A123 & A153) are checked prior to final inspection. The thickness of the Zinc (Zn) layer on the centrifuged hot-dip galvanized samples was measured before and after a corrosion test. The corrosion resistance of the samples was evaluated via the ASTM B117 salt spray test in a 5% (w/v) NaCl solution at 35 °C with 50% humidity for 10 days. The samples were removed and photographed at intervals of 2, 6, 8 and 10 days. The surface morphology and composition of the corrosion products were examined via a JEOL Quanta FEG 250 scanning electron microscope equipped with an energy dispersive X-ray spectroscopy (EDX) detector for elemental analysis.
Results and Discussion
Accelerated corrosion evaluation: ASTM B117 standard
Salt spray testing is a method used to simulate a corrosive environment and accelerate the natural process of corrosion. By exposing samples to a saline fog in a controlled chamber, this test helps to determine how well materials or coatings can withstand prolonged exposure to harsh conditions, such as those found in marine or industrial environments. It is typically used to assess the quality and durability of protective coatings, metals and other materials that may be exposed to saltwater or saltwater. The accelerated corrosive attack produced during the salt spray test allows rapid comparisons between the expected and actual corrosion resistance. Table 1-3 shows the changes in the physical appearance of the steel hooks at different time intervals when subjected to the salt spray test.
Table 1:Changes in the visual morphology of the surface of CS hooks after exposure to salt spray test for specific time intervals.

Table 2:Changes in the visual morphology of the surface of SS hooks after exposure to salt spray test for specific time intervals.
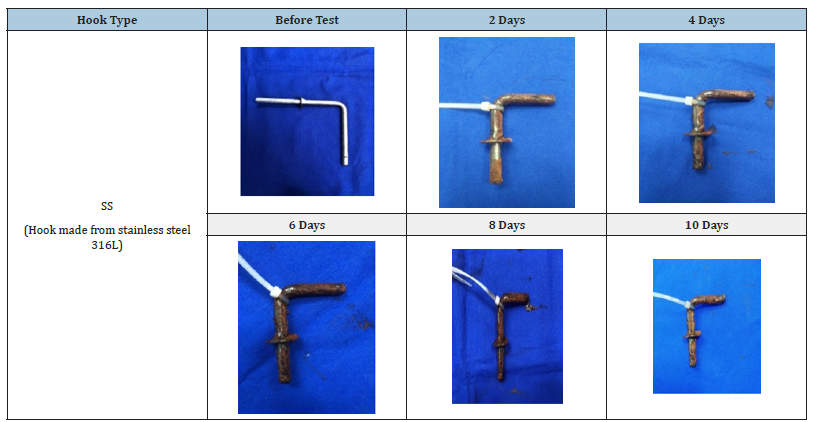
Table 3:Changes in the visual morphology of the surface of SHDG hooks after exposure to salt spray test for specific time intervals.
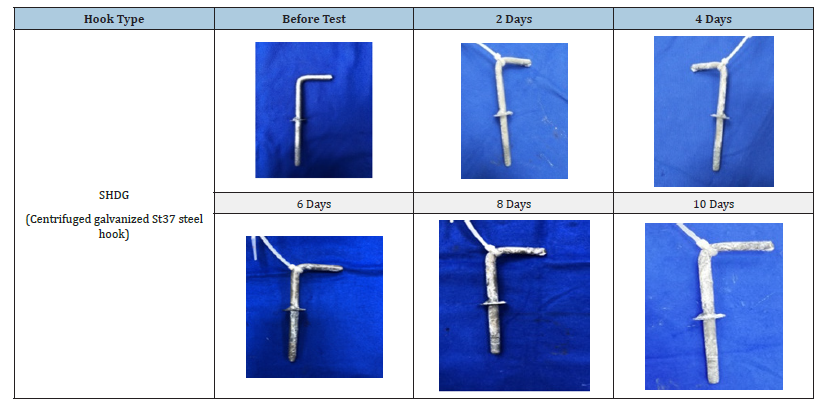
The phases and morphologies of the corrosion products were then analysed after the salt spray corrosion test. The degree of coverage and compactness of the surface corrosion products gradually increased from 2 to 10 days in the salt spray environment. The CS, SS and SHDG hooks were subjected to accelerated corrosion in a salt spray chamber for intervals of 2, 4, 6, 8 and 10 days. Under short-term corrosive conditions, the surface corrosion products consisted mainly of a brown loose, porous layer of flocculent γ-FeOOH. With increasing time, the corrosion products gradually changed to a lumpy and blocky dense structure of β-FeOOH and α-FeOOH [23]. The CS hooks corroded the most, and after corrosion in a saline environment, brown rust (Fe3O4) developed on the surface after corrosion in a saline environment [24]. The corrosion products of 316 L stainless steel or SS in NaCl can include metallic sulfates, NaHSO4 and multilayer interfacial corrosion oxides. These oxides may consist of an inner layer rich in chromium and an outer layer rich in sodium and iron. In general, SS is considered to be more corrosion resistant than CS. It is more resistant to pitting corrosion and has the ability to self-repair the passive film of oxides and hydroxides [25]. However, this depends on the specific situations and environments of galvanized or Stainless Steel (SS) parts. In the present study, the SS hooks also corroded but not to the same extent as the CS hooks did. Interestingly, the SHDG hooks presented no signs of brown rust. Only the deposition of a white patina of ZnO/Zn(OH)2 was observed on all hooks, which appeared to increase with increasing exposure time of the hooks.
SEM–EDX analysis of the hooks
The speculation about the nature of the corrosion product formed on the surface of the hooks was confirmed by the SEM micrographs obtained. EDS was used for qualitative and quantitative measurements, elemental distribution mapping and chemical state analyses of the samples.
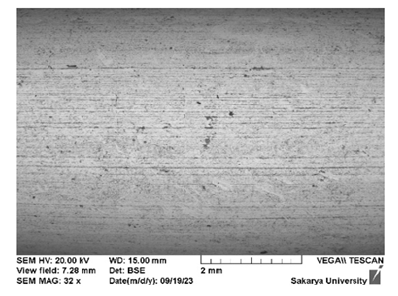
The surface morphology of polished SS before the salt spray test: The polished sample showed lines of abrasion on the SS hooks, and as the hooks had not been subjected to salt spray testing, a smooth morphology could be observed throughout (Figure 3).
Figure 3:SEM micrographs of a polished SS hook.
The surface morphology of the SS after the salt spray test: The SEM images of the SS sample surface obtained after the salt spray test revealed a rough and tarnished appearance of the SS sample surface. Uniform corrosion was observed along the lines of abrasion, as shown in Figure 4(a). An obvious increase in the elemental percentages of Fe and O was observed (Figure 4c & Table 4). Interestingly, the salt spray test also resulted in the formation of pits on the SS surface (Figure 4b).
Figure 4:SEM micrographs of the SS hook surface after the salt spray test.
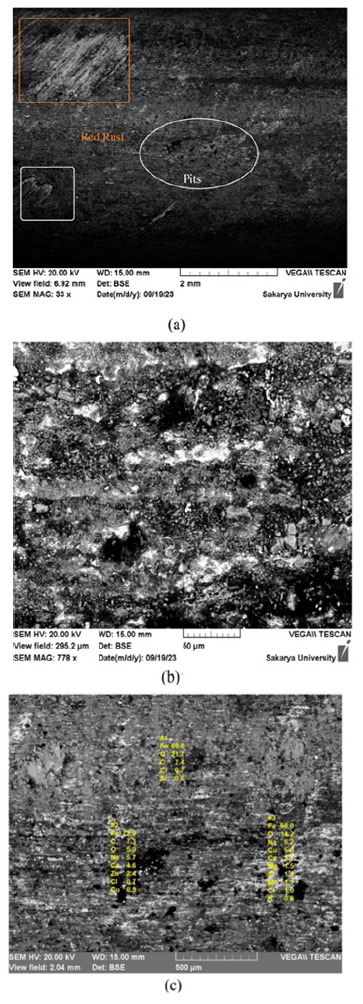
However, it is generally accepted that SS 316L steel has high resistance to pitting in chloride-rich environments. A protective passive layer of formed corrosion products was observed on the surface of the SS hook. To understand the corrosion of SS in the high-chloride environment of the test, elemental mapping was carried out around the pits to confirm the accumulation of Cl- ions, which are normally responsible for pitting in SS (Figure 5a-5c). The EDX evaluation revealed that after salt fogging, an increase in the elemental percentage was observed not only for Fe and O but also for Cl, which likely seeped into the pits formed from the test solution (Figure 5c & Table 4).
Figure 5:SEM-EDX evaluation of the corrosion products after rusting with the SS hook.

Table 4:Quantitative changes observed via EDX in steel hooks after salt fogging.
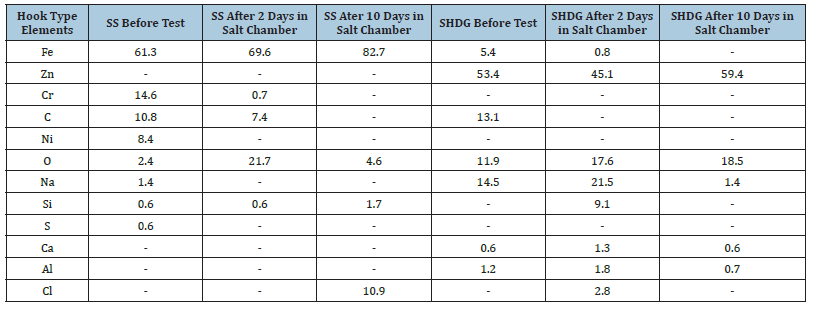
Figure 6:SEM micrograph of the surface of an SHDG hook showing the galvanized layer.

The morphology of the corrosion of the SHDG after the salt spray test: The micromorphology of the SHDG surface corrosion products after the salt spray corrosion cycles is shown in Figure 6. In fact, the density, thickness, and adhesion of the corrosion product layer are closely related to the corrosion kinetics of hot-dip galvanized steel. When the corrosion time is 2 days, a small amount of corrosion product is produced on the surface, but the corrosion product layer is discontinuous and cannot fully cover the entire galvanized layer.
The corrosion products on the surface of the hot-dipped galvanized steel increased and covered the entire surface of the galvanized layer. There was a small number of convex corrosion products, and the pits were distributed randomly after being subjected to salt fog. At the corrosion time of 10 days, the corrosion products generated were denser. This means that as the corrosion time increased, the barrier protection provided by the corrosion products on the surface of the hot-dip galvanized steel on the surface layer increased, and the corrosion resistance of the hot-dip galvanized steel also increased. The same outcome was observed after inspection of (Figure 7a-7c).
Figure 7:SEM micrographs of corrosion products formed on the surface of SHDG hooks on the 4th day of salt fogging.

The corrosion products produced at different corrosion stages are relatively similar. Most widely plated hydroxide and some needle-shaped ZnO cover the surface of the SHDG surface, as shown in Figure 8(a). Zinc hydroxides, which are shaped like a wide plate, precipitate on the surface of the zinc layer and cover most of the surface of the galvanized layer [26]. A comparison of the contents of various elements revealed that the main corrosion product of hot-dipped galvanized steel was zinc hydroxide, which has a better corrosion protection effect on the surface layer than does ZnO (Figure 8). When the salt fog test was conducted for 10 days, the corrosion resistance was attributed mainly to oxides and hydroxides formed on the surface. In normal structures in marine environments, the particles formed on galvanized steel further convert into zinc carbonates after reacting with atmospheric CO2 for several days. The quantitative results of the EDX are shown in Table 4. The contents of Fe and C decreased over the course of salt spray analysis, whereas those of Zn and O are gradually increased. This ruled out the possibility of carbonate formation. Hence, the white rust or zinc particles present after 10 days were mostly zinc hydroxides. This layer provided excellent protection against corrosion.
Figure 8:SEM micrographs for evaluating the surface of the corrosion products of SHDG on the 10th day of salt.
Conclusion
This scientific study provides further evidence that unprotected
hooks should be strictly avoided in GFRC panel installation as
they risk compromising structural integrity and durability due to
corrosion. The use of hot dipped galvanized hooks is essential to
ensure proper load distribution, corrosion prevention and meeting
safety standards.
a. While almost no corrosion was observed visually, in
micrographs or in elemental maps of the SHDG hooks, the
stainless-steel hooks showed red rust after only two days in
the salt chamber, followed by significant pitting as the test
duration increased. In general, stainless-steel hooks offer
superior corrosion resistance compared to St37 steel hooks.
However, under the aggressive conditions of the salt spray test,
the passive layer of the stainless steel is easily compromised,
particularly by abrasion during the cutting of the specimen for
SEM analysis, resulting in rapid corrosion.
b. Centrifugal hot-dip galvanization provides excellent
protection against corrosion for small parts, maintaining the
necessary fit accuracy of the hooks. Compared to the classic hotdip
galvanizing process, the zinc bath temperature in centrifugal
galvanization is substantially higher, ranging from a normal 450
°C to approximately 530 °C. This higher temperature leads to
improved drainage characteristics of zinc from the surface.
c. The duration of protection after hot-dip galvanizing is
highly dependent on atmospheric, mechanical, chemical, and
thermal influences. The hardness of the iron-zinc alloy layer
is significantly greater than that of typical construction steels,
making it much more resistant to mechanical stresses such as
impacts, shocks, and abrasion that may occur during transport,
assembly, or use.
d. The zinc layer thickness is generally particularly strong in
edge areas due to the chemical bond between steel and zinc,
providing optimal protection even in corrosion-prone areas
and cavities. However, if minor damage to the zinc surface
occurs, the chemical reactions between the steel, zinc, and
moisture will ensure that the exposed steel remains protected
by the surrounding zinc.
e. No rust forms beneath the zinc layer in the surrounding
areas. Because alloyed layers of zinc and iron are formed during
galvanizing, which are permanently bonded to the steel surface,
the adhesion is excellent. The hot-dip galvanizing process is
highly resource-efficient and environmentally friendly in all
aspects.
Acknowledgement
This study is a collaboration effort of academia and industry. The authors would like to express their gratitude to Beni Kohen, Volkan Özdal, Faik Ali Birinci, Sedat Enveş, Volkan Akmaz and Yasemin Hatipoğlu from the Fibrobeton R&D Centre for their valuable assistance throughout this research.
References
- Davalos MR, Van DWJ, Krawczyk B, Engelberg DL (2020) Corrosion behaviour of type 316L stainless steel in hot caustic aqueous environments. Metals and Materials International 26(5): 630-640.
- Rahbar RA, Zakeri AH (2011) Mechanical properties and corrosion resistance of normal strength and high strength steels in chloride solution. Journal of Naval Architecture and Marine Engineering 7(2): 94-100.
- Loukil M, Hassine WB, Limam O, Kotronis P (2019) Experimental determination of GFRC tensile parameters from three-point bending tests using an analytical damage model. Construction and Building Materials 223: 477-490.
- Sabat L, Soumya PS (2020) Experimental evaluation of a composite material using GFRC. Materials Today: Proceedings 21: 1330-1334.
- Özdal V, Maraşli M, Gerengi H, Dikmen K (2023) Corrosion behavior of embedded pad hook in glass fiber reinforced concrete. Civil Engineering Design 5(2): 18-25.
- Coşkun K, Gerengi H, Maraşlı M, Birinci FA, Özdal V (2023) Use of corrosion inhibitors in stainless steel anchors used in glass fiber reinforced concrete (GRC) precast facade elements. The European Journal of Research and Development 3(1): 1-15.
- Naidu GG, Joseph SA (2022) Corrosion behavior of fiber-reinforced concrete-A review. Fibers 10(5): 38.
- Marasli M, Dikmen K, Özdal V, Akmaz V, Gerengi H (2021) Investigation of the corrosion behavior of hot dip galvanized (HDG) St37 steel in 3.5% NaCl solution, 8th International Congress on Engineering, Architecture and Design, Oral presentation/Oral presentation pp. 681-690.
- Gerengi H, Maraşlı M, Rizvi M, Kohen B, Taşkıran I (2024) Protection of steel hooks embedded in glass-fiber-reinforced concrete against macrocell corrosion. Petroleum Research 9(2): 317-326.
- Gerengi H, Solomon MM, Maraslı M, Kohen BB (2022) A comparative analysis of the corrosion characteristics of electro-galvanized steel coated with epoxy zinc-free and zinc-rich coatings in 5% NaCl. Journal of Adhesion Science and Technology 37(20): 1-12.
- (2024) 10 Real benefits of galvanized steel.
- Toklu E, Gur M, Celtik M (2018) Investigation on effects of steel surface properties on galvanization behavior. Journal of Engineering Research and Applied Science 7(1): 861-868.
- Jain R, Pitchumani R (2018) Fabrication and characterization of zinc-based superhydrophobic coatings. Surface & Coatings Technology 337: 223-231.
- Natesan M, Venkatachari G, Palaniswamy N (2006) Kinetics of atmospheric corrosion of mild steel, zinc, galvanized iron and aluminium at 10 exposure stations in India. Corrosion Science 48(11): 3584-3608.
- Seré PR, Culcasi JD, Elsner CI, Di SAR (1997) Factors affecting the structure of zinc coating’s structure. Journal of Metallurgy 33(6): 376-381.
- Lin CS, Meshii M (1994) The effect of steel chemistry on the formation of Fe-Zn intermetallic compounds of galvanneal-coated steel sheets. Metallurgical and Materials Transactions B 25(5): 721-730.
- Feliu S, Barranco V (2003) XPS study of the surface chemistry of conventional hot-dip galvanised pure Zn, galvanneal and Zn-Al alloy coatings on steel. Acta Materialia 51(18): 5413-5424.
- Liu YW, Wang ZY, Cao GW, Cao Y, Huo Y (2017) Study on corrosion behavior of zinc exposed in coastal-industrial atmospheric environment. Materials Chemistry and Physics 198: 243-249.
- Saeedikhani M, Wijesinghe S, Blackwood DJ (2019) Revisiting corrosion protection mechanisms of a steel surface by damaged zinc-rich paints. Corrosion 75(7): 756-770.
- Maraşli M, Akmaz V, Kam M, Di̇kmen K, Gerengi̇ H (2022) Investigation of the effect of electro galvanizing process on the corrosion mechanism of St37 anchor element. Duzce University Science and Technology Journal 10(1): 367-378.
- Saeedikhani M, Wijesinghe S, Blackwood DJ (2020) Moving boundary simulation and mechanistic studies of the electrochemical corrosion protection by a damaged zinc coating. Corrosion Science 163: 108296.
- Gerengi H, Marasli M, Solomon MM, Coksun K, Guner Y (2024) Corrosion of centrifuged hot dip galvanized pad hooks used in GFRC Panels. Protection of Metals and Physical Chemistry of Surfaces 60(1): 1113-1119.
- Liu Y, Gao H, Wang H, Tao X, Zhou W (2024) Study on the corrosion behavior of hot-dip galvanized steel in simulated industrial atmospheric environments. International Journal of Electrochemical Science 19(1): 100445.
- Wang J, Jiang B, Cao J (2020) Corrosion mechanism of Q235A under 3.5% NaCl salt spray. Materials Transactions 61(12): 2342-2347.
- Nie J, Wei L, Jiang Y, Li Q, Luo H (2021) Corrosion mechanism of additively manufactured 316 L stainless steel in 3.5 wt.% NaCl solution. Materials Today Communications 26: 101648.
- Permeh S, Lau K (2023) Corrosion of galvanized steel in alkaline solution associated with sulfate and chloride ions. Construction and Building Materials 392: 131889.
© 2025 Marziya Rizvi. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






