- Submissions

Full Text
Progress in Petrochemical Science
Enhanced Stability and Performance of Hydrogenated Styrene–Butadiene Rubber (HSBR) for High-End Applications
Sudeep Dey, Arnab Dutta, Ajay C*, Sanjit Kumar Das, Mridul Dasgupta, Saikat Dasgupta, Barun Kumar Samui and Rabindra Mukhopadhyay
Hari Shankar Singhania Elastomer and Tyre Research Institute, India
*Corresponding author:Ajay C, Hari Shankar Singhania Elastomer and Tyre Research Institute, Mysore-570016, Karnataka, India
Submission: April 11, 2025;Published: May 22, 2025

ISSN 2637-8035Volume7 Issue 2
Abstract
Styrene-Butadiene Rubber (SBR) is a synthetic copolymer of styrene and butadiene, widely used across various industrial sectors due to its favorable mechanical and processing characteristics. However, the presence of residual carbon–carbon double bonds within the polybutadiene segments contributes to oxidative and thermal degradation, limiting its long-term performance. This study focuses on the hydrogenation of SBR latex to reduce unsaturation and enhance stability. The Hydrogenated SBR (HSBR) was evaluated in gum compounds without the addition of fillers or anti-degradants to assess the intrinsic polymer properties. Comparative studies were conducted using Natural Rubber (NR), Emulsion SBR (ESBR), and Solution SBR (SSBR). The SSBR, synthesized via anionic polymerization, exhibits controlled unsaturation, while ESBR is produced through free radical polymerization. NR, with its inherently high unsaturation, served as a benchmark. HSBR demonstrated superior mechanical and curing performance relative to the other rubbers. These improvements are attributed to the controlled hydrogenation process and the absence of natural proteins and other impurities. These results suggest HSBR can effectively replace conventional rubbers in high-performance applications, especially for enhanced wear properties in tires when compounded with reinforcing fillers such as carbon black or silica.
Keywords:Hydrogenated Styrene Butadiene Rubber (HSBR); Catalyst; Gum compound; Vulcanization; Mechanical properties; Thermal stability
Introduction
SBR is one of the most widely used synthetic elastomers due to its excellent balance of physical properties, ease of processing, and cost-effectiveness. However, its structural limitation lies in the residual unsaturation present in the butadiene segments of the polymer chain. These carbon–carbon double bonds are highly susceptible to degradation upon exposure to oxygen, ozone, and Ultraviolet (UV) radiation, particularly under elevated thermal conditions. This inherent vulnerability significantly restricts the durability of SBR in critical applications. To overcome this challenge, hydrogenation is employed to reduce the level of unsaturation in the polymer backbone, thereby improving its oxidative and thermal stability. HSBR represents a new generation of elastomers that combine the mechanical performance of traditional rubbers, such as natural rubber, emulsion SBR, and solution SBR, with enhanced environmental resistance. Despite these benefits, the hydrogenation process is often associated with high cost and complexity, particularly when using pressurized hydrogen gas or non-catalytic di-imide reduction techniques in solution [1]. These methods necessitate precise control and costly infrastructure. Catalytic hydrogenation, therefore, has emerged as a more efficient approach, utilizing catalysts such as RhCl(PPh₃)₃, Cp₂Co/BuLi, Ni/Al, Pd/Al₂O₃, and Ziegler–Natta systems. However, concerns over residual metallic impurities in the final product have led researchers to explore alternative catalysts, such as Ru(CH=CHPh)Cl(CO)(PCy₃)₂, which offers efficient hydrogenation at lower concentrations and with minimal contamination [2]. Pioneering work by Parker et al. has shown that HSBR displays enhanced thermal and oxidative resistance when subjected to extreme conditions. In their study, HSBR was analyzed under both aerobic and anaerobic environments up to 600 °C using thermogravimetric analysis, differential scanning calorimetry, sol-gel analysis, infrared spectroscopy, and Nuclear Magnetic Resonance (NMR). These investigations highlighted the role of antioxidants and hydrogenation levels in determining the degradation pathways and stability of the rubber [3]. Such findings underscore the relevance of hydrogenation in extending the service life of rubber-based materials. This study evaluates HSBR alongside NR, ESBR, and SSBR in unfilled gum compounds. By excluding fillers, anti-degradants, and other additives, the focus remains solely on the base polymers, allowing for a direct assessment of their inherent properties. The goal is to establish whether HSBR can serve as a viable replacement for conventional rubbers in applications requiring high durability and performance under thermal or oxidative stress, as well as superior ozone resistance.
Key Results and Discussion
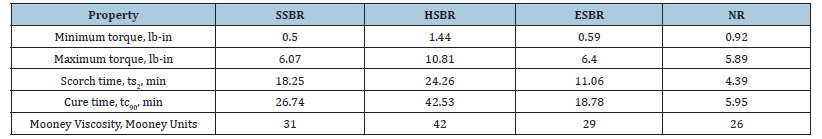
All rubber compounds were prepared using a conventional melt-mixing process, followed by sulfur-based vulcanization. Zinc Oxide (ZnO) and stearic acid were used as activators for the crosslinking process. The curing characteristics were evaluated by rheological analysis, and mechanical properties were measured post-curing to assess compound performance. The scorch time (ts₂), which indicates the processing safety window before vulcanization begins, varied significantly among the rubbers. NR exhibited the shortest ts₂ value at 4.4 minutes, suggesting a high risk of premature curing during processing because of the existence of many natural proteins and fatty acids, whereas HSBR mixed composites showed the longest ts2 at 24.3 minutes. HSBR contributes the prolonged mixing with high stability due to the non-appearance of natural impurities. ESBR and SSBR showed intermediate values of 7.5 and 11 minutes, respectively. The optimum cure time (tc₉₀) followed a similar trend, HSBR mixed composites showing 700% higher time as compared with NR based composites to reach 90% cure (Table 1). The fast cure in NR can be attributed to the presence of higher sulfur reactivity and it increased with the presence of uncontrolled unsaturation, while the delayed cure in HSBR reflects its saturated backbone and more controlled crosslinking behavior. ESBR and SSBR again exhibited intermediate cure times, consistent with their polymerization processes and partial unsaturation levels. Mooney viscosity (ML 1+4 @ 100 °C), an indicator of the compound’s processability and green strength, was highest in HSBR (Table 1). This elevated viscosity indicates stronger molecular interactions and better dimensional stability in the unvulcanized state, a desirable feature for rubber production. The Mooney viscosity is beneficial for good filler dispersion and low energy consumption. Optimum Mooney viscosity also contributes for better green strength and gives better shape during compression molding process. The increased viscosity was a consequence of hydrogenation, which reduced segmental motion by eliminating double bonds and restricting chain rotation [4].
Table 1:Rheometric Properties of different types of gum compounds
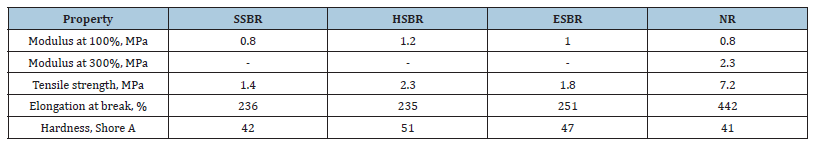
In terms of mechanical performance, HSBR-based compounds demonstrated almost 50% higher 100% modulus compared to NR indicating improved stiffness and reinforcement (Table 2). This behavior can be attributed to stronger polymer-polymer interactions and the absence of non-rubber constituents such as proteins, lipids, and resins found in NR. While NR showed a high 300% modulus due to extensive sulfur crosslinking and natural tackiness, HSBR offered a more balanced and predictable mechanical profile (Table 2). The tensile strength of NR was significantly higher (7.2 MPa) compared to HSBR (2.3 MPa), a result expected given NR’s superior green strength and elasticity (Table 2). However, the higher hardness observed in HSBR-approximately 10 units more than the other compounds-demonstrates its rigid structure and resistance to deformation, a result of the limited chain mobility introduced by hydrogenation [5].
Table 2:Physical properties of different types of gum compounds.
Overall, the results confirm that HSBR delivers a balanced performance across critical parameters such as curing behavior, processability, stiffness, and thermal stability. Although its tensile strength is lower than that of NR, its enhanced hardness, scorch safety, and Mooney viscosity make it suitable for high-performance rubber applications. Furthermore, the absence of natural impurities and the controlled hydrogenation process contribute to consistent behavior, reinforcing its potential as a substitute for traditional rubbers in carbon black- or silica-filled compounds.
References
- He Y, Daniels ES, Klein A, El AMS (1997) Hydrogenation of styrene‐butadiene rubber (SBR) latexes. Journal of Applied Polymer Science 64(10): 2047-2056.
- Pan Q, Garry LR (2004) Hydrogenation of styrene‐butadiene rubber catalyzed by Ru(CH=CHPh)Cl(CO)(PCy3)2. Macromolecular Rapid Communications 25(8): 843-847.
- Sarkar MD, Mukunda PG, Prajna PD, Anil KB (1997) Degradation of hydrogenated styrene-butadiene rubber at high temperature. Rubber Chemistry and Technology 70(5): 855-870.
- Dutta A, Chanda J, Bhandary T, Pal A, Das SK, et al. (2021) Utilization of modified soybean oil in passenger car radial tyre tread and sidewall compound to promote green mobility. Intl J Sci Research Publications 11(6): 432-448.
- Hemil K, Arnab D, Hirak S, Tirthankar B, Sanjit KD, et al. (2022) Use of coal based black in tyre inner liner and tube compound: A new sustainable end use application. Polymer Sci peer Rev J 3(5): 1-13.
© 2025 Ajay C. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






