- Submissions

Full Text
Progress in Petrochemical Science
Production of High Purity Lignin from OPEFB: An Overview
Jofry Othman*, Norliza Abd Rahman, Jarinah Mohd Ali and Siti Kartom Kamarudin
Department of Chemical and Process Engineering, Faculty of Engineering and Built Environment, Universiti Kebangsaan Malaysia, Malaysia
*Corresponding author:Jofry Othman, Department of Chemical and Process Engineering, Faculty of Engineering and Built Environment, Universiti Kebangsaan Malaysia, Malaysia
Submission: April 23, 2024;Published: May 01, 2024

ISSN 2637-8035Volume6 Issue2
Abstract
This study examines the practicable method of lignin delignification and fractionation from Oil Palm Empty Fruit Bunches (OPEFB) to produce high purity products. A review on the current literature progress in this field indicated two development phases that involves several early studies on lignin delignification and lignin product purification and the later phase provides initial attempts to improve lignin recovery efficiency and overcome technical barriers for obtaining high purity lignin. It is found that there are significant gaps in understanding to achieve efficient removal of lignin, good recovery of lignin from high purity products and the removal of silica residuals on OPEFB surfaces. Accordingly, the high technical barriers to achieve high-purity lignin products are identified, including the need for an improved method to delignify and fractionate lignin for the purpose of producing a higher purity product. It can be concluded that fractions of lignin may be segregated and isolated by using the ultrafiltration method based on molecule mass and recovery to a higher purity product with over 98wt.%. High purity applications of OPEFB lignin, such as the propylene stabilizer and chemical sunscreen products, can therefore be produced. Accordingly, this review also provided an opportunity to further develop product applications towards anti-ultraviolet, antioxidant and antibacterial functions.
Keywords:Delignification; OPEFB; Lignin fractionation; Ultrafiltration
Introduction
The high-value lignin applications require high-purity fractions that necessitate an efficient lignin recovery method from Oil Palm Empty Fruit Bunches (OPEFB). Major difficulties have arisen because of the complex nature of OPEFB polymer, which has been encountered in prior attempts to produce highly purity lignin. Lignin is a phenol-based polymer abundantly found in the cell wall of OPEFB and acts as an insoluble three- dimensional network [1]. It also has huge potential to be used in many applications for anti-ultraviolet, antioxidant and antibacterial functions besides its primary function of cell strength and as an adhesive on plant composite materials [2]. Due to these properties, lignin has attracted attention and offers additional advantages in terms of its ability to pick up radicals and inhibition of oxidative stress. In view of its high lignin content, OPEFB that is traditionally considered a waste product in plantation industries, has emerged as a valuable source for valorizing lignin into numerous usages. In addition, its usefulness as a sustainable stable of lignin is also supported by the large availability of OPEFB in plantation areas and their role as primary palm waste. The fiber structure of OPEFB is very fibrous and contains a great potential as a raw material for various synthesis approached.
OPEFB has a fibrous structure, irregular shape and weighs about 3.5kg. It is currently used in limited quantities in personal care products [3,4] has provided a typical composition of OPEFB at 51.88% cellulose and hemicelluloses, 31.68% lignin and 6.69% ash percentage. However, the development of effective delignification is made particularly challenging by the complicated nature of OPEFB polymers consisting in a complex mixture of physical and chemical characteristics [1]. This paper examines the progress on development of delignification method from OPEFB, which can attain high purity lignin products. In particular, the efficient removal of lignin, the good recovery of lignin to high purity lignin products, and the removal silica residuals on the EFB surface, are the acceptable delignification performance.
Overview on the Lignin Delignification Process
To obtain closer to the native lignin properties, the viability of the lignin delignification process will have to overcome the recalcitrance of OPEFB. Therefore, to ensure that the target lignin composition and properties are obtained, appropriate extraction and delignification methods must be used. According to Siahkamari et al. [5], lignin is an amorphous polyphenolic material arising from an enzyme-mediated dehydrogenates polymerization of three major phenylpropanoid monomers, which are coniferyl, sinapyl and p-coumaril alcohol. The lignin structural elements are linked by carbon-carbon and ether bonds to form a tri- dimensional network associated with the hemicellulos’s polysaccharides inside the cell wall [6]. Lignin is a phenol-based polymer abundantly found in the cell wall of OPEFB and acts as an insoluble three- dimensional network [1]. Specifically, it requires an optimized pretreatment method at different rates and temperatures to achieve controlled composition of extracted lignin. The recalcitrance of lignocellulose is mainly because lignin functions as a physical barrier and hemicellulose reduces the pore size of the biomass sample preventing the access of cellulose [7].
The extraction approach of lignin and cellulose from OPEFB is analyzed in such a way as to dissolve the lignocellulose complex into a possible individual component. To obtain closer to the native lignin properties, the viability of the lignin delignification process will have to overcome the recalcitrance of OPEFB. Therefore, to ensure that the target lignin composition and properties are obtained, appropriate extraction and delignification methods must be used. According to Siahkamari et al. [5], lignin is an amorphous polyphenolic material arising from an enzyme-mediated dehydrogenates polymerization of three major phenylpropanoid monomers, which are coniferyl, sinapyl and p-coumaril alcohol. The lignin structural elements are linked by carbon-carbon and ether bonds to form a tri-dimensional network associated with the hemicellulos’s polysaccharides inside the cell wall [6]. In addition, Siahkamari et al. [5] investigated the complexity of lignin as a heterogeneous polymer, plant cell walls and the characterization of phenolic monolayers. The viability of the delignification method will have to overcome the recalcitrance of OPEFB, for extracting it closer to the native lignin properties. In particular, the efficient removal of lignin, the good recovery of lignin to target composition, the removal of spikes of silica on the EFB surface, which acts as a physical barrier to hydrolysis, are acceptable criteria for the delignification method [8]. OPEFB’s lignin extraction methods include a delignification process that cleaves ether bonds to remove lignin and alkaline hydrolysis to remove hemicellulose, lignin and other impurities such as oils, cuticles, etc. [3]. It is therefore necessary to separate lignin into monomers with low molecular weight given its high macromolecular complexity before they can be used in the targeted products. Accordingly, high-value applications for lignin require high-purity fractions, that also co-produce lowpurity or intermediates lignin for other applications such as usage in activated carbon.
Previous Research on High Purity Lignin from OPEFB
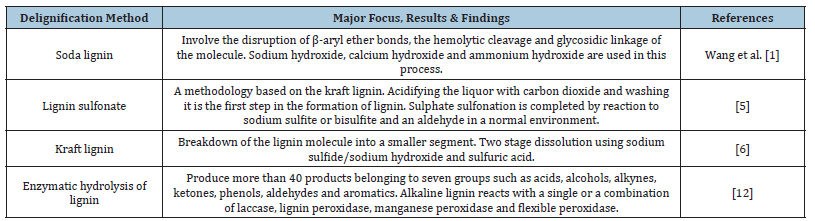
It was suggested by Mohammad et al. [6], that OPEFB is extracted by means of a variety of methods, i.e., with sulphuric acid, alkaline and lignin sulfonate method in the process of lignin extraction. Chemical pretreatments with either acid, base or other catalysts have a robust and reasonable digestion of OPEFB resulting in varying results for specific lignin fragments from the crude lignin molecule. According to Mohammad et al. [6], this method usually involves the disruption of β-aryl ether bonds, the hemolytic cleavage and glycosidic linkage of the molecule. Lignin bonds are broken, the solubility and removal of lignin is improved by alkali pretreatments, but lignin recovery is difficult [8]. However, Ajayi et al. [3] found that excessive fiber digestion will occur if the concentrations of caustic soda are higher than optimal condition and may result in fiber damage. Therefore, optimization of this method can potentially improve from the previous results on residual sugar content of 1.5 to 3.0wt%, ash content of 0.7 to 2.3wt.% and moisture content of 2.5 to 5.0wt.%. This in turn can lead to achieving this method could be capable of producing lignin to a higher of lignin purity.
In the pulping industry, black liquor was extracted from 5 lignocellulosic wastes using sodium hydroxide at temperatures of up to 160 C with pressure between 10 and 12 bar [2]. The method of extracting lignin from black liquor produced during OPEFB pretreatment may include acid precipitation and membrane separation [9]. Perera et al. [9] applied sequential acid and alkali pretreatment to generate a lignin recovery of 68.9% by using 2.5wt.% caustic soda at 121 °C and with 80-minute reaction time. Under less severe conditions, acids can degrade lignin-cellulose, but they require expensive processing equipment such as acidresistant stainless steels, coatings or high-temperature inhibitors. Therefore, alkaline pretreatment is increasingly being pursued as chemical delignification and removal of hemicellulose from OPEFB. This method saponifies intermolecular ester linkages that crosslink lignin and hemicellulose. It causes lignocellulosic materials to swell, which reduces the degree of polymerization and crystallinity of cellulose while increasing the surface area of lignocellulosic materials [10]. Alkaline pretreatment removes lignin and hemicellulose saponification of the cross-linking intermolecular ester bonds between them [7]. This method breaks lignin bonds, improves lignin solubility and removal, but lignin recovery is difficult. However, if the NaOH concentration is higher than the optimal condition, excessive fiber digestion will occur, leading to fiber damage. Therefore, efficient delignification requires a significant reaction time and can be performed at low temperature and pressure.
Another method is based on a two-step acidification method using caustic soda and sulfuric acid, prior to being processed in the separation unit. The is to maintain a balance of sodium and Sulphur in chemical synthesis, through exchanging lignin sodium, by hydrogen for high sodium recovery rate and lignin having a low ash content [2]. According to Yiamsawas et al. [11] injection of carbon dioxide gases acidifies the black liquor that liberates lignin particle into the suspension phase. The suspended is then filtered, suspended into aqueous sulfuric acid, prior to the final filtration and drying into pure lignin product [11]. Accordingly, this method is currently able to produce lignin with lower average molecular weight but also produces undesirable methoxy, phenolic and ash impurities in the lignin product [2]. The next approach uses lignin sulfonate that is derived from kraft lignin. The lignin is first precipitated by acidifying the liquor with carbon dioxide prior to washing. Then the reaction with sodium sulfite or sodium bisulfite and an aldehyde in a basic environment result in complete sulfonation, i.e. This approach can produce sulfonated lignin, in which the sulfonic acid groups will form on an aromatic ring as opposed to aliphasic sidechains i.e. dimethyl sulfite and dimethyl sulfoxide [5]. However, according to Wang and Lee [1] the attainable lignin purity is low due to the high impurities present in sulfur at 3.5-8.0wt.%, ash at 4.5-8.0wt.% and moisture at 5.8wt.%.
The enzymatic hydrolysis of lignin is an emerging approach that shows moderate lignin degradation rate of 22-29% with low energy consumption, mild reaction conditions, and environmental friendliness [12]. According to Mastrolitti et al. [2], this method may result in up to 40 products of lignocellulose belonging to the seven groups described below, such as acids, alcohols, alkanes, ketones, phenols and aromatics. Specifically, alkaline lignin reacts with single or combination of laccase, lignin peroxidase, manganese peroxidase and versatile peroxidase. This produces lignin of medium to high purity containing sulfur from 0-1.0wt.%, ash at 1.0-3.0wt.% and moisture at 4.0-9.0wt.% [12]. However, for high purity applications such as propylene stabilizers and chemical sunscreen, the development of suitable enzymatic strains for OPEFB is required. A summary of the methods to delignify OPEFB, with key results and findings is set out in Table 1. Additionally, Mondylaksita et al. [7] suggested that lignin monomers possess radical scavenging abilities which can neutralize reactive oxygen species and protect cells from oxidative damage. Several studies were carried out at an early proof of concept stage to explore the potential applications of OPEFB lignin in food and other industries by means of a couple of possible extraction methods. Perera et al. [9] has explored the antioxidant capacity of lignin, attributing its efficacy to the presence of phenolic compounds within its structure. This potential for antioxidant activity makes lignin a promising candidate to be used in pharmaceutical, food preservation and other industries where oxidative stability is of vital importance. Another study by Ajayi et al. [3] was conducted on the antioxidative property of lignin obtained from OPEFB, which demonstrated that a comparative importance could be shown for functional groups between extracted lignin and commercially available lignin with regard to its purity as derived from black liquor. The possible suitability of lignin properties as antioxidants is in functioning as a sustainable and effective solution for oxidative stress-related issues. The radicals, which are made because of normal cell processes or brought into the body by other factors like pollution and ultraviolet radiation, are extremely reactive molecules [9]. Free radicals, which are linked with ageing and other chronic diseases, can cause oxidative stress if we do not control them [3]. The appeal of lignin to a variety of industrial uses is enhanced not only by its antioxidative properties but also by its ecological and renewable nature. The summary of the methods is tabulated in Table 1.
Table 1:OPEFB delignification method.
Initial Concept of High Purity Lignin Production
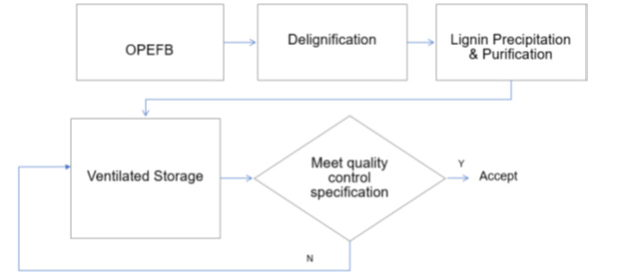
From literature, studies on lignin delignification from OPEFB for high purity application for MEG production can be segregated into two development phases, namely? and? As shown in Figure 1, the lignin degradation concept includes OPEFB pretreatment for delignification, lignin fractionation, precipitation and purification into products. The first phase involves several early studies on lignin delignification and lignin product purification that are based on various approaches from alkali pretreatment and acid precipitation for product recovery, alkali pretreatment with carbon dioxide acidification, alkali pretreatment with oxidation and carbon dioxide acidification and solvent based pretreatment using deep eutectic solvent. From the literature analysis, the focus of those studies was largely on OPEFB feedstock pretreatment and lignin degradation aspects but did not consider methods for obtaining high purity lignin. The second phase of the lignin delignification phase further improve form the initial OPEFB delignification concept but added new techniques to improve lignin recovery efficiency and overcome technical barriers of EFB lignin to high purity applications for obtaining high purity lignin. This includes the use of a combined mechanical and physiochemical pretreatment, enzymatic approach including gasification-based acidification. As opposed to the initial concept of OPEFB delignification, the second phase of lignin degradation improves including new techniques to improve lignin recovery efficiency and overcome technical barriers of EFB lignin to high purity applications for obtaining high purity lignin. This shall include the use of a combination of mechanical and physiochemical pretreatments, including an enzymatic process for gasification acidification.
Figure 1:Lignin pretreatment from OPEFB into high purity product.
Modified Lignin Delignification and Precipitation
The use of a highly alkaline solution at pH 13.0, which causes long term corrosion problems, low lignin recovery of 59%, generation of a large volume of wastewater and impurities in products, has a negative performance impact [8]. This is in addition to the low biomass loading of chemical solvents at a ratio of 1 to 9. The improvement of this method, while enabling the structure of lignin fiber for other cellulosic applications to be maintained, is therefore necessary for achieving a high yield of soluble lignin. A study by Yiamsawas et al. [11] on the alkali pretreatment with carbon dioxide acidification, suggested that this approach can achieve lignin having a low ash content. These authors proposed that to increase the filterable capacity, carbon dioxide injection should be used to control a lesser black liquor pH of less than 10 that was found to promote lignin coagulation. Next, Mastrolitti et al. [2] have analyzed the alternative approach to using alkali pretreatment with oxidation and followed by carbon dioxide acidification. It was suggested that alkali pretreatment using oxidation by blowing oxygen into the liquor at 75 °C in combination with lignin precipitation using carbon dioxide can generate improved lignin slurry composition. The lignin slurry can then be agitated, coagulated at moderate temperature & filtered using a filter press to generate a sulfur-free quality lignin [7].
Therefore, based on the review, this study proposes that an improved process be introduced for the fractionation and extraction of lignin to take account of these limitations. The conceptual process is based on a milder alkali delignification of fresh OPEFB with lignin precipitation using gaseous carbon dioxide, pH compensation to match strain requirement and enzymatic fractionation. In a controlled molecular weight, the fractionated lignin mixture may have an enhanced composition, closer to its native structure and in a suitable lignin complex for the downstream processing for higher lignin purity and finer particle sizes. A mild lignin black liquor makes it possible for microbial strains to be adequately enzymatically synthesized to facilitate the higher yield and environmentally friendly recovery of lignin. In addition, the unconverted black liquor that contains sodium carbonate and water can be upgraded into by- products in cleaning agent and detergent.
Lignin Fractionation and Purification into High Purity
It is expected that the fractionated lignin will have a crude lignin purity of at least 90wt.% and that it will have a better coagulation to be subjected to a more refined separation process. It is therefore possible to separate and isolate fractions of lignin by means of the ultra-filtration method based on molecular weight. After molecular weight isolation into a fine fraction, recovery to a high purity product of more than 98wt.% is possible. For applications of high purity lignin, for example in the use of propylene stabilizers and chemical sunscreens where pure lignin has been incorporated as an ingredient into each product formulation, then it is appropriate. Measurements of the molecular weight, hydroxyls content, ash contents, polydispersity index and viscosity are part of the characterization tests to be performed on the produced lignin [5]. Measurement needs to be carried out on the use of chemical sunscreen with an ultraviolet blocking capacity, antioxidant activity, radical scavenging characteristics and color evaluation. In the case of unmodified and modified forms, where a blend with at least 10% additional compounds is not required, testing results shall be used to qualify lignin. Accordingly, with the improved purity of the produced lignin, further reduction into nanoparticles is possible through the various known techniques such as polymerization, physical process or cross-linked [13]. For establishing eligible lignin in both modified and unmodified forms, with a blend below 10% that includes other substances, the test results shall be taken into account. The final lignin products will produce lower color as a result of controlling the particle size and decreasing the polydispersity index [14-23].
Conclusion
This overview concluded that there is a practicable method of lignin delignification and fractionation which can be used to produce high purity products. In an analysis of literature progress in developing a OPEFB delignification method it appears that there are gaps in understanding to achieve efficient removal of lignin, good recovery of lignin from high purity products and the removal of silica residuals on OPEFB surfaces. This is due to the high technical barriers to achieving high-purity lignin products for high-value applications that necessitate an efficient lignin recovery method from OPEFB. Major difficulties have arisen because of the complex nature of OPEFB polymer that acts as an insoluble three-dimensional network. A method to delignify and fractionate lignin for the purpose of producing a higher purity product is suggested. It indicates that, using the ultra-filtration method based on molecule mass and recovery to a higher purity product with more than 98wt.%, lignin fractions can be separated and isolated. For instance, in use of propylene stabilizers and chemical sunscreen, it is therefore possible to produce OPEFB lignin for high purity applications. Therefore, for example in the use of propylene stabilizers and chemical sunscreens, it is possible to mature OPEFB lignin to high purity applications. Furthermore, the results of this study also provide an opportunity to further develop product applications for anti-ultraviolet, antioxidant and antibacterial functions.
Conclusion
Solid oxide fuel cells and electrolysers stand as beacons of hope in our quest for a sustainable future. Beyond their scientific intricacies, they embody a convergence of innovation and belief, where the boundaries between science and spirituality blur. As we unravel the mystique of these marvels of modern science, let us embrace the new perspectives, recognizing that true progress lies not only in technological advancement but also in the evolution of consciousness.
References
- Wang W, Lee DJ (2021) Lignocellulosic biomass pretreatment by deep eutectic solvents on lignin extraction and saccharification enhancement: A review. Bioresource Technology 339: 125587.
- Mastrolitti S, Borsella E, Guiliano A, Petrone M, Bari I (2021) Sustainable lignin valorization technical lignin, processes and market development. IEA Bioenergy.
- Ajayi SM, Olusanya SO, Sodeinde KO, Didunyemi AE, Atunde MO, et al. (2023) Hydrophobic modification of cellulose from oil palm empty fruit bunch: Characterization and application in pickering emulsions stabilization. Carbohydrate Polymer Technologies and Applications 5: 100282.
- Shanmugarajah B, Chew IM, Mubarak NM, Choong TS, Yoo C, et al. (2019) Valorization of palm oil agro-waste into cellulose biosorbents for highly effective textile effluent remediation. Journal of Cleaner Production 210: 697-709.
- Siahkamari M, Emmanuel S, Hodge DB, Nejad M (2022) Lignin-glyoxal: A fully biobased formaldehyde-free wood adhesive for interior engineered wood products. ACS Sustainable Chem Eng 10(11): 3430-3441.
- Mohammad IN, Ongkudon CM, Misson M (2020) Physicochemical properties and lignin degradation of thermal-pretreated oil palm empty fruit bunch. Energies 13(22): 5966.
- Mondylaksita K, Ferreira JA, Millati R, Budhijanto W, Niklasson C, et al. (2020) Recovery of high purity lignin and digestible cellulose from oil palm empty fruit bunch using low acid-catalyzed organosolv pretreatment. Agronomy 10(5): 674.
- Chia SM, Chiong MC, Panpranot J, Lee KM (2023) Process optimization on co-production of lignin and cellulose in deep eutectic solvent pretreatment of oil palm empty fruit bunch. Biomass Conversion and Biorefinery 1-10.
- Perera UP, Foo ML, Chew IM (2023) Synthesis and characterization of lignin nanoparticles isolated from oil palm empty fruit bunch and application in biocomposites. Sustainable Chemistry for Climate Action 2: 100011.
- Rashid T, Taqvi SA, Sher F, Rubab S (2021) Enhanced lignin extraction and optimisation from oil palm biomass using neural network modelling. Fuel 293: 120485.
- Yiamsawas D, Watcharin K, Pongprayoon T (2023) Enhanced performance of lignin recovery with a carbon dioxide acidification method. ACS Omega 8(8): 7438-7444.
- Iram A, Berenjian A, Demirci A (2021) A review on the utilization of lignin as a fermentation substrate to produce lignin- modifying enzymes and other value-added products. Molecules 26(10): 2960.
- Gao K, Liu J, Li X, Gojzewski H, Sui X, et al. (2022) Lignin nanoparticles as highly efficient, recyclable emulsifiers for enhanced oil recovery. ACS Sustainable Chem 10(29): 9335-9339.
- Haqiqi MT, Bankeeree W, Lotrakul P, Pattananuwat P, Punnapayak H, et al. (2021) Antioxidant and UV-blocking properties of a carboxymethyl cellulose- lignin composite film produced from oil palm empty fruit bunch. ACS omega 6(14): 9653-9666.
- Hidayat M, Aqilah NA, Winata A (2022) Pretreatment of oil palm empty fruit bunch using caustic soda solution for lignin isolation. Journal of Applied Science and Engineering 25(6): 1025-1030.
- Lee SC, Yoo E, Lee SH, Won K (2020) Preparation and application of light-colored lignin nanoparticles for broad-spectrum sunscreens. Polymers 12(3): 699.
- Rahman MS, Mondal MI, Yeasmin MS, Sayeed MA, Hossain MA, et al. (2020) Conversion of lignocellulosic corn agro- waste into cellulose derivative and its potential application as pharmaceutical excipient. Processes 8(6): 711.
- Riaz T, Zeeshan R, Zarif F, Ilyas K, Muhammad N, et al. (2018) FTIR analysis of natural and synthetic collagen. Applied Spectroscopy Reviews 53(9): 703-746.
- Sadeghifar H, Ragauskas A (2020) Lignin as a UV light blocker-a review. Polymers 12(5): 1134.
- Suota MJ, Silva TA, Zawadzki SF, Sassaki GL, Hansel FA, et al. (2021) Chemical and structural characterization of hardwood and softwood LignoForce™ Industrial Crops and Products 173: 114138.
- Wang W, Guo T, Sun K, Jin Y, Gu F, et al. (2019) Lignin redistribution for enhancing barrier properties of cellulose-based materials. Polymers 11(12): 1929.
- Yang W, Qi G, Kenny J, Puglia D, Ma P (2020) Effect of cellulose nanocrystals and lignin nanoparticles on mechanical, antioxidant and water vapour barrier properties of glutaraldehyde crosslinked PVA films. Polymers 12(6): 1364.
- Zhang H, Fu S, Chen Y (2020) Basic understanding of the color distinction of lignin and the proper selection of lignin in color-depended utilizations. International Journal of Biological Macromolecules 147: 607-615.
© 2024 Jofry Othman. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






