- Submissions

Full Text
Progress in Petrochemical Science
Simulation of the Pure Hydrogen Production and CO2 Capture with the Calcium Looping Process in the Combustion of Coal
Azam Movasati1 and Hassan Ghanbarabadi2*
1 Chemical, Petroleum and Gas Engineering Department, Iran University of Science and Technology, Iran
2 Department of Chemical Engineering, Technical and Vocational University (TVU), Iran
*Corresponding author:Hassan Ghanbarabadi, Department of Chemical Engineering, Technical and Vocational University (TVU), Jajarm Branch, Jajarm, Iran
Submission: July 06, 2018;Published: July 13, 2018

ISSN 2637-8035Volume2 Issue3
Abstract
It is widely accepted today that global concentration of carbon dioxide (CO2) in the atmosphere is increasing rapidly. The main method for separating this gas is the mineral carbonation process. In this process, hydrogen-rich stream is produced by the removal of CO2. A feasibility simulation study was conducted to evaluate the utilization of CaO used in the combustion of coal processing plant. The fluidized bed reactor of calcium looping process was simulated by Aspen Plus software. In addition, the evaluated of the energy and economic process was carried out with Aspen Energy Analyzer and Aspen Economics Analyzer, respectively. The results of the simulation show that the hydrogen purity can reach more than 84vol% and more than 98.96vol% of CO2 along with combustion of coal is captured with calcium looping process. The results of the study with Aspen Economics Analyzer show that the operating cost of the process is about 914350$/year and the cost of utilities is about 5508.4$/h.
Keywords: CO2 capture; Hydrogen; CaO; Fluidized bed reactor; Aspen plus
Introduction
figure 1: Diagram description of the coal-CaO-steam process.
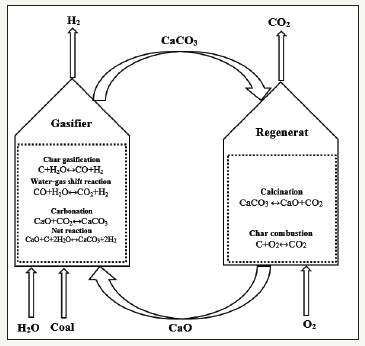
The emission of carbon dioxide is due to the use of fossil fuels. Among the types of fuels, coal has the highest concentration of CO2 emissions [1,2]. Increasing the concentration of CO2 causes many problems, including drought, acidification of ocean water and global warming [2,3]. One of the methods of CO2 capture is the use of the calcium looping process [4]. In this process was carried out the carbonation-calcinations reaction that carbon dioxide gas from coal combustion is separated and pure hydrogen is produced [5,6]. Research shows for the carbonation-calcinations reaction has shown that initially the rate reaction is rapid and chemically controlled, but then undergoes a sudden transition to a slower diffusion controlled regime. Therefore, the carbonationcalcinations reaction can commence with a regime of the residence time of the solids chemical and diffusion controlling step then followed by a pure diffusion controlling regime. The reaction rate, that with increasing the residence time of the solids, more oxygen from CaO took part in the reaction with CO2 gas which accordingly improved the reaction in the carbonator reactor [7]. The Coal-CaOsteam system consists of gasifier and regenerator reactors and the reactions of this process are shown in Figure 1 [8]. CO2 carbonation with CaO has the advantages such as: a) production of CO2 carbonate and ready for separation, b) removal of CO2 from the system, c) the production of hydrogen-rich gas stream, d) providing the required heat for coal gasification [5,9]. If the pure oxygen is used in the combustion reaction, the output flow from the regenerator reactor has a high concentration of CO2 that is separated and stored [10]. In this process, if the residence time of the solid material CaO in the carbonator is increased, absorption capability can be achieved. In this research, the CaO process was studied by proposing a developed model of the fluidized bed reactor in the Aspen Plus software. Energy and economic analysis were also conducted with Aspen Energy Analyzer and Aspen Economics Analyzer, respectively..
The Simulation Results
In the coal-CaO-steam system, there are the gasifier, the riser and the regenerator reactors [11]. A series of R Yield and R Gibbs reactors in the Aspen Plus software was considered as a fluidized bed reactor (Figure 2) [12]. In the Coal-CaO-steam system, the coal is initially introduced into the R Yield reactor and pyrolysis and decomposes at temperature of 1050 °C. Characteristics of coal and operational conditions can be found in Tables 1&2, according to reports of Chen S et al. [4]. Simulation of the R Yield reactor has been carried out with the Calculator and Fortran sections of the Aspen Plus software. The Gibbs reactor was used to carry out equilibrium reactions. The production rate was calculated in the reactor output stream, using the minimum Gibbs free energy system. The simulation results of Gasifier, Riser and pyrolysis reactors are presented in Table 3.
figure 2:Flow diagram of carbonation-calcinations process conditions modelled in Aspen Plus.
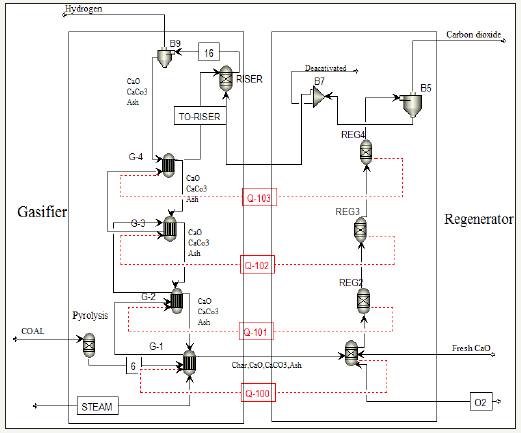
Table 1: Characteristics of coal [4]. Lower heating value (LHV fuel) =26.81MJ/kg.
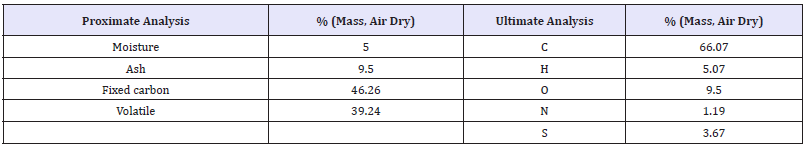
Table 2: Operational conditions for Coal-CaO-steam system.
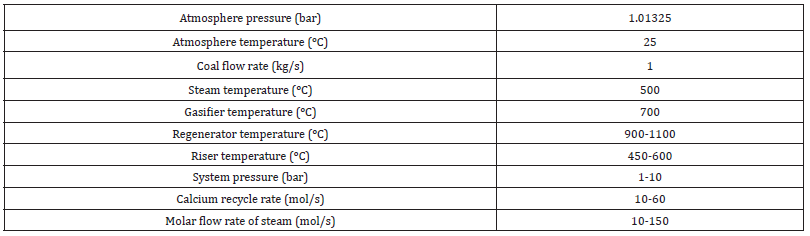
Table 3: Outlet gas compositions for the Gasifier, Riser and pyrolysis cases simulated.
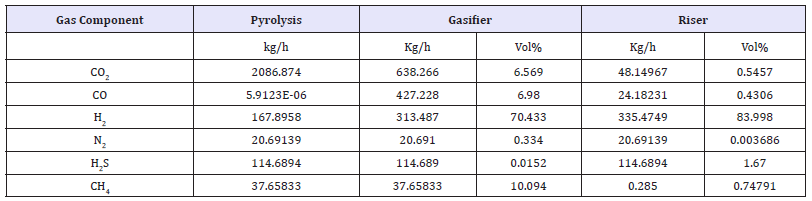
figure 3:The effect of the steam flow rate input on hydrogen production case simulated.
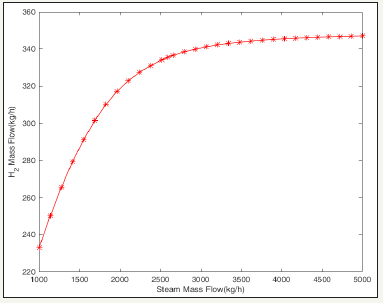
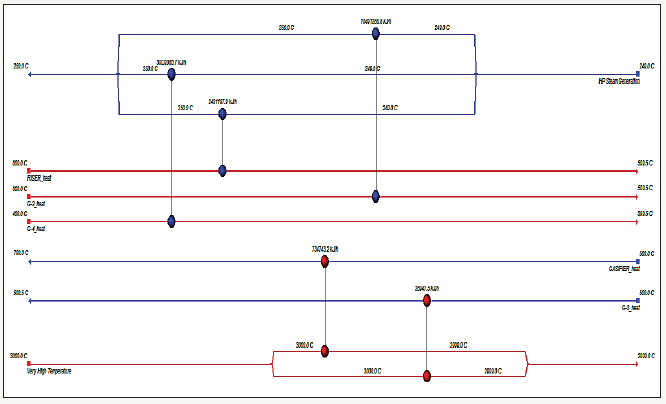
The simulation results are shown in Table 3 that more than 98.96vol% of CO2 along with combustion of coal is captured and the hydrogen purity can reach 84vol% at a steam flow 40mol/s and CaO recycle rate 30mol/s [4]. In addition, the simulation results show that increasing the steam flow rate will lead to more CO2 capture and increase H2 production (Figure 3) [9]. However, increasing the flow rate of the steam increases the system’s the operating costs of carbonation-calcinations process. The results, indeed, a suitable the steam flow rate is about 2594.00kg/h. The energy analysis of the Coal-CaO-steam system was carried out with the Aspen Energy Analyzer (Figure 4). The simulation results indicate that the entire energy requirement of the Coal-CaO-steam system is 4.635+3kw. In addition, economic analysis with Aspen Economics Ananlyzer shows that the operating cost of the system is about 914350$/year and the cost of ethyl iodine is about 4.5508$/h [10].
figure 4:Aspen Energy Analyzer flow sheet of the simulation.
Conclusion
In this work, calcium looping process simulation was carried out for production of pure hydrogen and carbon dioxide absorption from coal combustion. The fluidized bed reactor used in this process was developed with the Aspen Plus software. The development of fluidized bed model was carried out from one reactor to eight reactors (gasifier and regenerator). The results show that CO2 emission is about 2086.874kg/h from 3600kg/h of coal combustion. The results show that more than 98.96vol% of CO2 along with combustion of coal is captured and the hydrogen purity can reach 84vol% at a steam flow 40mol/s and CaO recycle rate 30mol/s. The calcium looping process has many advantages over CO2 absorption with chemical solvents. Among the advantages of this process is the high capacity of calcium oxide, good ability to remove CO2 and pure H2 production, which can be used in various industries.
References
- Léonard G, Lepaumier H, Thielens ML, Toye D, Heyen G (2012) CO2 capture in power plants: process simulation and solvent degradation. Journée Energie GEPROC.
- Khoshandam B, Kumar RV, Allahgholi L (2010) Mathematical modeling of CO2 removal using carbonation with CaO: The grain model. Korean Journal of Chemical Engineering 27(3): 766-776.
- Léonard G (2013) Optimal design of a CO2 capture unit with assessment of solvent degradation. LGC Chem Eng.
- Chen S, Wang D, Xue Z, Sun X, Xiang W (2011) Calcium looping gasification for high-concentration hydrogen production with CO2 capture in a novel compact fluidized bed: Simulation and operation requirements. International Journal of Hydrogen Energy 36(8): 4887-4899.
- Han L, Wang Q, Yang Y, Yu C, Fang M, et al. (2011) Hydrogen production via CaO sorption enhanced anaerobic gasification of sawdust in a bubbling fluidized bed. International Journal of Hydrogen Energy 36(8): 4820-4829.
- Hanak DP, Biliyok C, Anthony EJ, Manovic V (2015) Modelling and comparison of calcium looping and chemical solvent scrubbing retrofits for CO2 capture from coal-fired power plant. International Journal of Greenhouse Gas Control 42: 226-236.
- Ortiz C, Chacartegui R, Valverde JM, Becerra JA (2016) A new integration model of the calcium looping technology into coal fired power plants for CO2 capture. Applied Energy 169: 408-420.
- Connell DP, Lewandowski DA, Ramkumar S, Phalak N, Statnick RM, et al. (2013) Process simulation and economic analysis of the Calcium Looping Process (CLP) for hydrogen and electricity production from coal and natural gas. Fuel 105: 383-396.
- Hanak DP, Manovic V (2017) Calcium looping combustion for highefficiency low-emission power generation. Journal of Cleaner Production 161: 245-255.
- Cormos CC (2014) Economic evaluations of coal-based combustion and gasification power plants with post-combustion CO2 capture using calcium looping cycle. Energy 78: 665-673.
- Hawthorne C, Trossmann M, Galindo Cifre P, Schuster A, Scheffknecht G (2009) Simulation of the carbonate looping power cycle. Energy Procedia 1(1): 1387-1394.
- Aspen Technology I (2011) Aspen Physical Property System (Physical Property Methods), Burlington, USA.
© 2018 Hassan Ghanbarabadi. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






