- Submissions

Full Text
Orthopedic Research Online Journal
A Comparative Study of Infliximab and its Biosimilar: Efficacy and Safety in Rheumatoid Arthritis and Ankylosing Spondylitis
Hanan Abushwereb1*, Hayat Sherif1, Basma Al Habash2 and Rajab Tarsin2
1Pharmacology and Clinical Pharmacy Department, University of Tripoli, Libya
2Pediatric Oncology Department, Tripoli Medical Center, Libya
*Corresponding author:Hanan Abushwereb, Pharmacology and Clinical Pharmacy Department, University of Tripoli, Faculty of Pharmacy, Tripoli, Libya
Submission: June 17, 2024;Published: July 01, 2024

ISSN: 2576-8875 Volume11 Issue1
Abstract
Background: Rheumatoid Arthritis (RA) and Ankylosing Spondylitis (AS) are two chronic autoimmune diseases for which the infliximab biosimilar CT-P13 has received regulatory approval for use in all indications of the reference medication.
Objectives: This study was conducted to assess the effectiveness and safety of biological infliximab versus its infliximab biosimilar administered to Libyan adult patients with RA and AS.
Methods: : This retrospective observational study was conducted on 135 patients with confirmed RA and 72 patients with AS. The patient groups were subdivided into those treated with infliximab biosimilars and reference infliximab. Comparative analyses were conducted using t-tests to assess differences in disease activity.
Results: There were no appreciable changes in the safety and efficacy of the two therapies, and both RA and AS patients treated with infliximab biosimilar or reference infliximab had significant reductions in disease activity..
Conclusion: The safety and effectiveness profiles of both the infliximab biosimilar and reference infliximab in treating RA and AS are similar, recommending the use of or switching to the less expensive biosimilar.
Keywords:Rheumatoid arthritis, Ankylosing spondylitis, Infliximab, Infliximab Biosimilar, Safety
Introduction
Inflammatory rheumatic diseases are a heterogeneous group of autoimmune conditions such as Rheumatoid Arthritis (RA), Ankylosing Spondylitis (AS), and Psoriatic Arthritis (PsA) for which treatment is centered on immunosuppression, which was previously primarily based on steroids and other disease-modifying antirheumatic drugs (DMARDs) [1-3]. The development of targeted biological therapies, which are genetically engineered proteins derived from human genes that are specifically designed to inhibit specific immune system components that play critical roles in inflammation [4,5], is one of the most significant innovations in rheumatology, and its efficacy was demonstrated by better outcomes with longer remissions, fewer disabilities, and a higher quality of life [4]. The utilization of biological agents such as infliximab has revolutionized therapy, resulting in transformative changes in the treatment approaches for chronic and debilitating autoimmune disorders such as rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis, and inflammatory bowel diseases [5]. In addition, infliximab is a monoclonal antibody that is produced from human and mouse proteins by recombinant technology [6]. Monoclonal antibodies are proteins that recognize and bind to certain special proteins in the body [6]. Infliximab acts by neutralizing the biological activity of the pro-inflammatory cytokine tumor necrosis factor-alpha (TNF-α), preventing it from interacting with its receptors on the cell [6]. It prevents synovial and intestinal inflammation [7]. Although this advancement has improved clinical and public health outcomes in both the short and long term and has greatly enhanced patient care, these biological drugs are prohibitively expensive. In addition, because biological treatments are very expensive and only a few patients are being treated or have access to these effective treatments globally, long-term expenditure and costs have become unsustainable for payers and societies. Thus, the global sales of licensed original anti-tumor necrosis factor (anti- TNF) drugs (infliximab, etanercept, certolizumab, adalimumab, and golimumab) were estimated as a significant cost barrier to universal health care [8]. A biosimilar of reference infliximab (CTP13 or Remsima®), which was approved by the European Medicine Agency (EMA) and Food and Drug Administration (FDA) in 2013 and 2016, respectively, as a monoclonal anti-body that targets TNF [9] and has similar physiochemical characteristics to reference infliximab [10]. This new biosimilar CT-P13 has been used in the treatment of chronic inflammatory autoimmune diseases such as RA and AS to reduce the progression of joint erosion in RA and pain due to stiffness in AS [11]. CT-P13 shares similarities with infliximab in amino acid sequence, glycosylation profile, and structure, ensuring comparable binding affinity with TNF-α and inhibiting its pro-inflammatory activity [12]. However, not all patients well respond to these TNF inhibitors and need combination with other medications like methotrexate to enhance their effectiveness [13]. Moreover, TNF inhibitors may have a potential risk of exacerbating heart failure, a minimal risk of lymphoma and other injection side reactions, demyelinating disease, etc. [14]. Thus, this study aimed to assess the effectiveness and safety of biological infliximab versus its biosimilar CT-P13 in patients diagnosed with RA and AS. Comparing and evaluating the therapeutic outcomes, including symptoms, disease, and quality of life improvement, between the two available treatment options. Hence, the findings of this study will provide valuable insights on the management and treatment decisions taken to optimize patient care.
Methods
Study design
A retrospective observational study was conducted at Tripoli University Hospital on patients registered at the Rheumatology Department from February 2009 to September 2022. Data were collected from the medical records of patients in two groups diagnosed with Rheumatoid Arthritis (RA) and Ankylosing Spondylitis (AS). Both groups of patients were at least 12 weeks on either treatment at the time of recruitment in the current study. All patients were naïve to infliximab treatment at the start of treatment.
Study population
The Remsima® group for RA patients had n= 81 participants (17 males, 64 females), enrolled from December 2017 to May 2022. Meanwhile, the Remicade® group for RA patients included n=54 participants (10 males, 44 females), enrolled from February 2011 to September 2022. For AS patients, the Remsima® group (n= 36; 21 males, 15 females) from December 2017 to October 2021. While the Remicade® group (n-=36; 31 males, 5 females), enrolled from February 2009 to June 2022.
Medication source
This study has used reference infliximab (Remicade®) produced by Janssen Biologics, a subsidiary of Johnson & Johnson. Janssen Biologics is headquartered in Leiden, the Netherlands, and the biosimilar (Remsima®) manufactured by the company Celltrion Healthcare Co., Ltd. South Korean Hikma Pharmaceutical company. Taking into consideration ethics in research and data collection which was permitted by Tripoli University Hospital ethical and research committee.
Data collection
Data were collected from the medical records database, including demographic data such as age, sex, and body weight, as well as disease characteristics such as rheumatoid factor (RF) for RA patients, human leukocyte antigen B27 (HLA-B27) for AS patients, disease duration, duration of infliximab treatment, and use of disease-modifying antirheumatic drugs (DMARDs).
Disease assessment
DAS28 Calculator to calculate the disease activity score with a 28-joint count (DAS28). Taking into account inputs such as tender and swollen joint counts, the patient’s global assessment on a visual analog scale (VAS), and inflammatory markers such as ESR. The DAS28 score is a continuous measure ranging from 0 to 10, with higher scores indicating higher disease activity, and lower scores suggesting lower disease activity or remission [15].
Treatment assessment
Data was collected on treatment changes, reasons for changing, discontinuations, and reasons for discontinuation.
Safety test
Incidence, severity, duration, relation to infliximab treatment, and outcome of Adverse Events (AEs) recorded. Adverse events, commonly referred to as side effects, include infusionrelated reactions, (hypersensitivity and anaphylactic reactions), Tuberculosis (TB), latent TB, and serious infection.
Safety monitoring
Before starting infliximab, patients underwent a complete blood count, liver function tests, hepatitis screen, urine routine examination, and tuberculin test. These tests were requested to monitor potential adverse effects during follow-up.
Data Analysis
The data was analyzed using SPSS version 24, with variables expressed as frequencies, percentages, mean ± SD, standard deviation, and Standard Errors (SE). A comparative analysis between two medications using independent samples and a t-test was used to calculate the P-value and determine if there was a significant difference between the means of Disease Activity Score of 28 Joints (DAS28) and Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) based on the patient’s condition at the start of infliximab and the last follow-up. Paired sample t-tests were used to determine the effect of medication. The study also utilized chi-square tests to identify treatment type and EULAR response categories, and multiple linear regression analyses to examine factors impacting disease activity scores post-infliximab treatment for safety profile comparison. Statistical significance was determined by a P-value of less than 0.05, with a higher value indicating a higher level of significance.
Results
The study involved 135 patients with rheumatoid arthritis, divided into Remsima® and Remicade® treatments. Remsima® included 81 participants, 17 males and 64 females, while Remicade® included 41 participants, 6 males and 35 females. Both groups were divided into two groups for treatment. The CT-P13 treatment group (RA) had a higher percentage of female patients (79%, n = 64) than male patients (21%, n = 17) (Figure 1). Similarly, in the reference infliximab treatment group, there were more female patients (81.50%, n = 44) than male patients (18.50%, n = 10).
Figure 1:The gender ratio between CT-P13 and reference infliximab treatments in RA patients.
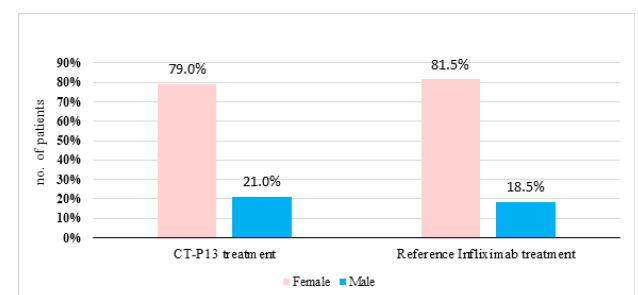
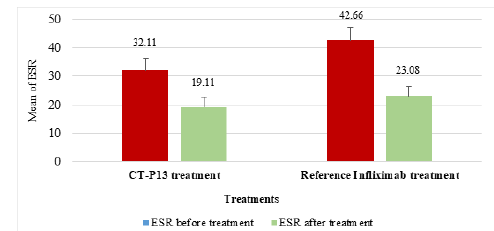
Figure 2 displays the gender distribution of patients in the CT-P13 and reference infliximab treatment groups (AS). The CTP13 group had more male patients (58.00%), while the reference infliximab group had more male patients (86.10%). This disparity was observed in both groups. The ESR reduction in RA patients was 22.29% with CT-P13, compared to 30.08% with the reference infliximab (Figure 3). Whereas, the ESR reduction in AS patients by CT-P13 was 40.48%, despite the reference infliximab showed a 45.89% reduction, indicating a lower ESR compared to CT-P13 treatment (Figure 4).
Figure 2:The gender ratio between CT-P13 and reference infliximab treatments in AS patients.
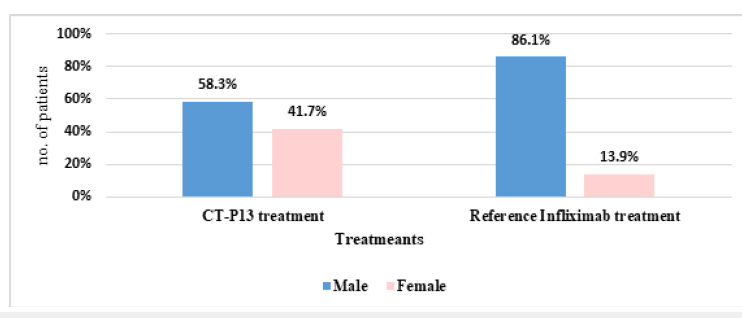
Figure 3:The mean ESR in RA patients before and after CT-P13 and reference infliximab treatments. (ESR: Erythrocyte Sedimentation Rate). EULAR: European League against Rheumatism.

Figure 4:The gender ratio between CT-P13 and reference infliximab treatments in RA patients.
CT-P13 and infliximab showed similar tender joint count in 28 joints (TJC28) reductions for RA patients, with a 52.25% reduction and 54.53% reduction respectively, indicating no significant differences (Figure 5). The SJC28 reduction for CT-P13 was 65.32%, while the reference infliximab showed a reduction of 78.72%, indicating a higher SJC28 reduction with infliximab (Figure 6). In contrast, the VAS reduction for CT-P13 was 65.87%, compared to 48.13% for the reference infliximab treatment in Figure 7, indicating a higher VAS by CT-P13 (Figure 7).
Figure 5:The means of TJC28 in RA patients before and after CT-P13 and reference infliximab treatments.
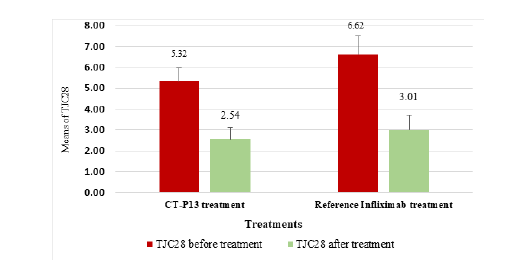
Figure 6:The means of SJC28 (Swollen Joints Count 28) in RA patients before and after CT-P13 and reference infliximab treatments.
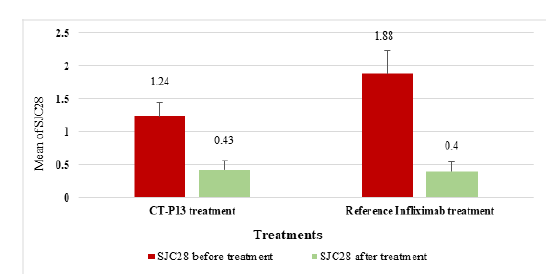
Figure 7:The mean VAS in RA patients before and after CT-P13 and reference infliximab treatments.
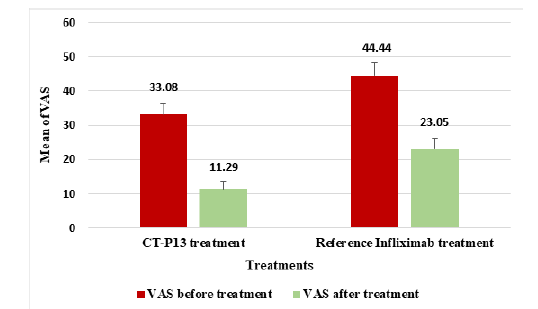
Figure 8:Compares the mean DAS28-ESR before and after CT-P13 and reference infliximab treatments in RA patients [15].
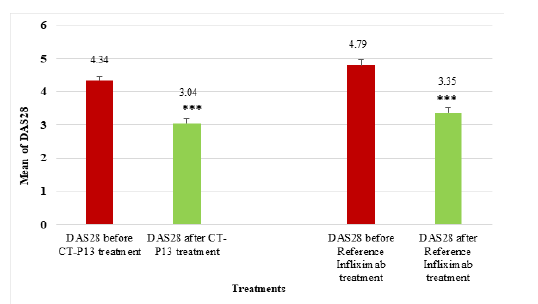
The mean DAS28 score for RA patients before CT-P13 treatment was 4.387, but after treatment, it decreased to 3.085. Similarly, the mean DAS28 score before infliximab treatment was 4.788, but after treatment, it decreased to 3.354 (Figure 8). The study found no significant difference between CT-P13 and reference infliximab groups, but significant differences before and after treatment within each group (P < 0.001). Moreover, the study found a significant improvement in the mean DAS28 score for 81 patients before and after treatment, with a mean decrease of 1.301. The data revealed a gender disparity with 17 male patients and 64 female patients. The treatment significantly improved the mean DAS28 score for male patients from 4.331 to 2.494, while the mean DAS28 score for female patients decreased from 4.339 to 3.179, indicating a statistically significant improvement.
The mean BASDAI score for AS patients before CT-P13 treatment was 4.901, which decreased to 2.581 post-treatment, and similarly for infliximab treatment, which decreased to 2.162 post-treatment (Figure 9). The CT-P13 and reference infliximab groups showed no significant difference before or after treatment, but there were significant differences within each group before and after treatment. The CT-P13 and reference infliximab treatment responses for RA and AS were compared (Figure 10 & 11), revealing variations in disease activity responses based on the European League against Rheumatism assessment.
Figure 9:Comparison of mean BASDAI before and after CT-P13 and reference infliximab treatments for AS patients.
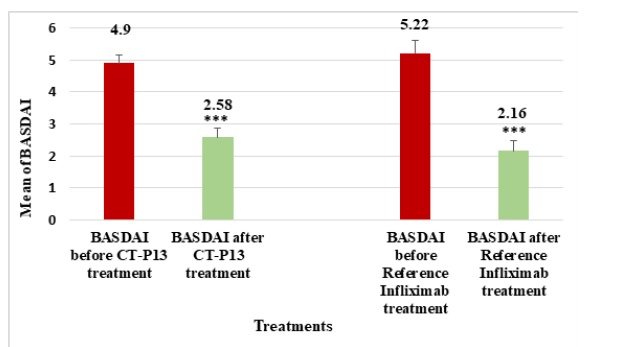
Figure 10:Disease activity response between CT-P13 and reference infliximab treatments by EULAR assessment in RA patients.
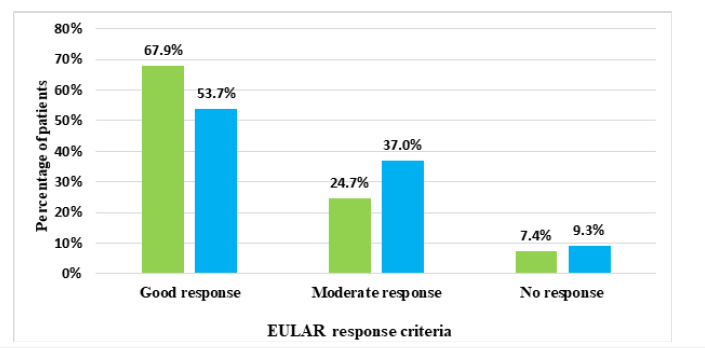
Figure 11:The EULAR assessment showed a significant difference in disease activity response between CT-P13 and reference infliximab treatments in AS patients.
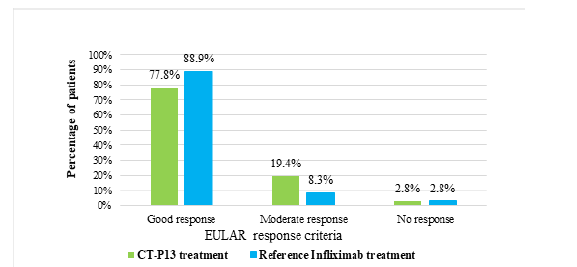
Figure 12 shows that the most common adverse effect was a Urinary Tract Infection (UTI), with 55.5% of CT-P13 patients experiencing it, compared to 22.2% of the infliximab group. The CT-P13 treatment group had a higher incidence of hypersensitivity reactions (19.4%) compared to the reference infliximab treatment group (8.3%) as shown in Figure 13.
Figure 12:The adverse effects observed in AS patients receiving CT-P13 and reference infliximab treatments.
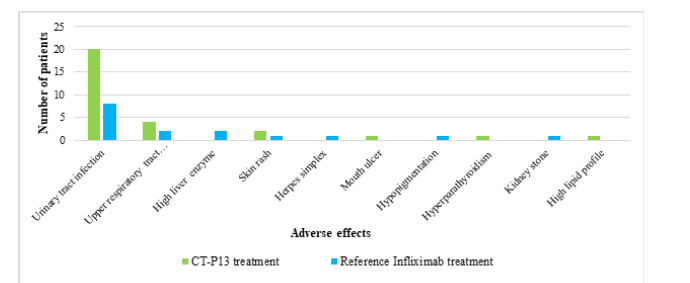
Figure 13:The percentage of hypersensitivity reactions observed in AS patients treated with CT-P13 compared to reference infliximab treatments.
Discussion
The results of this study provide valuable insights into management and treatment decisions made to improve patient care. After the World Health Organization approved the use of these biologic devices based on available global evidence and studies proving that there are no clinically meaningful differences between the biologic and the original drug in quality characteristics, biological activity, clinical effectiveness, safety, and immunogenicity. The results of this study are in line with previous studies in terms of effectiveness and degree of safety [16]. This study assesses the comparative effectiveness and safety of reference infliximab (Remicade®) and its biosimilar CT-P13 (Remsima®) in Libyan patients with RA and AS.
The study found that CT-P13 administration significantly reduced inflammation rate in RA and AS patients, with no significant difference between CT-P13 and reference infliximab groups, but significant differences before and after treatment [17,18]. Thus, CT-P13 and infliximab significantly improved clinical outcomes in patients with RA and AS, reducing disease activity scores. Infliximab’s mechanism of action involves binding to TNF-α, disrupting its interaction, reducing inflammation, suppressing immune cell migration, inducing cell death, and downregulating inflammatory cytokines [19,20], leading to alleviation of symptoms and improved physical capabilities [21]. CT-P13 and reference infliximab treatments significantly improved disease activity and patient quality of life in RA and AS patients, demonstrating the potential of biological therapies for managing symptoms [22]. Both CT-P13 and reference infliximab strongly decrease the mean DAS28 and BASDAI scores. This reduction indicates a significant improvement in disease activity and highlights the effectiveness of these therapies for managing symptoms and enhancing the quality of life for patients [23,24].
Urinary Tract Infection (UTI) was the primary adverse effect observed in CT-P13 and reference infliximab treatments for RA patients, affecting 43.2% and 37%, respectively. Both treatments showed similar safety profiles in managing RA, with slight variations in specific adverse effects, but overall differences were minimal. Bechman et al. [25] similarly mentioned the risk of infection with RA. Hypersensitivity reactions were more common in the reference infliximab group, while severe infusion reactions, pregnancy, and treatment failure were rare in both CT-P13 and reference infliximab groups. The majority of patients in both groups continued their medication without interruption, despite discontinuation being unrelated to adverse events or treatment failure. Similarly, UTI was the most common adverse effect in AS patients, occurring more in CT-P13 patients than those of reference infliximab patients, with slight differences between groups. Thus, CT-P13 and reference infliximab showed similar safety profiles for AS treatment, with slight differences in adverse effects between the groups. Hypersensitivity reactions were more frequent in the CT-P13 treatment group (19.4%) compared to the infliximab group (8.3%), indicating a higher risk of hypersensitivity. Previous studies have suggested a slightly higher risk of hypersensitivity with CT-P13 [26,27]. In summary, both treatments had similar safety profiles, with the most common adverse effect being Urinary Tract Infections (UTI). There was a slightly higher incidence of hypersensitivity reactions in the CT-P13 group.
Conclusion
Biosimilars provide lower-priced medicines, improving accessibility. Long-term studies monitor safety and effectiveness, and help doctors make informed treatment decisions. The results also support the use of biosimilars as a cost-effective alternative to original biologics. By addressing the proposed improvements, clinicians can better understand and manage these inflammatory rheumatic diseases..
References
- Wu F, Gao J, Kang J, Wang X, Niu Q, et al. (2022) Knowledge Mapping of exosomes in autoimmune diseases: A bibliometric analysis. Frontiers in Immunology 13.
- Smolen JS, Aletaha D, McInnes IB (2016) Rheumatoid arthritis. Lancet 388(10055): 2023-2038.
- Braun J, Sieper J (2007) Ankylosing spondylitis. Lancet 369(9570): 1379-1390.
- Feldmann M, Maini RN (2002) Discovery of TNF-α as a therapeutic target in rheumatoid arthritis: preclinical and clinical studies. Joint Bone Spine 69(1): 12-18.
- Thorne C, Bensen WG, Choquette D, Chow A, Khraishi M, et al. (2014) Effectiveness and safety of infliximab in rheumatoid arthritis: analysis from a Canadian multicenter prospective observational registry. Arthritis Care Res (Hoboken) 66(8): 1142-51.
- Rahman P, Choquette D, Bensen WG, Khraishi M, Chow A, et al. (2016) Biologic Treatment Registry Across Canada (BioTRAC): a multicentre, prospective, observational study of patients treated with infliximab for ankylosing spondylitis. BMJ Open 6(4): e009661.
- Wong M, Ziring D, Korin Y, Desai S, Kim S, et al. (2008) TNFalpha blockade in human diseases: mechanisms and future directions. Clin Immunol 126(2): 121-136.
- Peng K, Blais JE, Pratt NL, Guo JJ, Hillen JB, et al. (2023) Impact of introducing infliximab biosimilars on total infliximab consumption and originator infliximab prices in eight regions: An interrupted time-series analysis. Bio Drugs 37(3): 409-420.
- Gabbani T, Deiana S, Annese V (2017) CT-P13: design, development, and place in therapy. Drug Des Devel Ther 11: 1653-1661.
- An Q, Zheng Y, Zhao Y, Liu T, Guo H, et al. (2019) Physicochemical characterization and phase I study of CMAB008, an infliximab biosimilar produced by a different expression system. Drug Des Devel Ther 13: 791-805.
- Ward MM, Deodhar A, Gensler LS, Dubreuil M, Yu D, et al. (2019) 2019 update of the american college of rheumatology/spondylitis association of america/spondyloarthritis research and treatment network recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Care and Research 71(10): 1285-1299.
- Braun J, Kudrin A (2015) Progress in biosimilar monoclonal antibody development: The infliximab biosimilar CT-P13 in the treatment of rheumatic diseases. Immunotherapy 7(2): 73-87.
- Sammaritano LR, Bermas BL, Chakravarty EE, et al. (2020) 2020 American college of rheumatology guideline for the management of reproductive health in rheumatic and musculoskeletal diseases. Arthritis Rheumatol 72(4): 529-556.
- Li J, Zhang Z, Wu X, Zhou J, Meng D, et al. (2021) Risk of adverse events after anti-TNF treatment for inflammatory rheumatological disease. A Meta-Analysis. Frontiers in Pharmacology 12: 1-13.
- Anderson JK, Zimmerman L, Caplan L, Michaud K (2011) Measures of rheumatoid arthritis disease activity: Patient (PtGA) and Provider (PrGA) global assessment of disease activity, Disease Activity Score (DAS) and Disease Activity Score with 28-Joint Counts (DAS28), Simplified Disease Activity Index (SDAI), Cl. Arthritis Care and Research 63(SUPPL. 11): 14-36.
- Meyer A, Rudant J, Drouin J, Coste J, Carbonnel F, et al. (2019) The effectiveness and safety of infliximab compared with biosimilar CT-P13, in 3112 patients with ulcerative colitis. Aliment Pharmacol Ther 50(3): 269-277.
- Yoo DH, Hrycaj P, Miranda P, Ramiterre E, Piotrowski M, et al.(2013) A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis 72(10): 1613-1620.
- Yoo DH, Prodanovic N, Jaworski J, Miranda P, Ramiterre E, et al. (2017) Efficacy and safety of CT-P13 (biosimilar infliximab) in patients with rheumatoid arthritis: comparison between switching from reference infliximab to CT-P13 and continuing CT-P13 in the PLANETRA extension study. Ann Rheum Dis 76(2): 355-363.
- Hirohata S (2000) Rheumatoid arthritis, malignant rheumatoid arthritis. Ryōikibetsu Shōkōgun Shirīzu 59(29 Pt 4): 265-268.
- Shinde A, Tang X, Singh R, Brindley DN (2024) Infliximab, a monoclonal antibody against TNF-α, Inhibits NF-κB Activation, autotaxin expression and breast cancer metastasis to lungs. Cancers 16(1): 52.
- Placha D, Jampilek J (2021) Chronic inflammatory diseases, anti-inflammatory agents and their delivery nanosystems. Pharmaceutics 13(1): 64.
- Kim HA, Lee E, Lee SK, Park YB, Lee YN, et al. (2020) Retention rate and safety of biosimilar CT-P13 in rheumatoid arthritis: data from the korean college of rheumatology biologics registry. Bio Drugs 34(1): 89-98.
- Fautrel B, Bouhnik Y, Dieude P, Richette P, Dougados M, et al. (2023) Real-world evidence of the use of the infliximab biosimilar SB2: data from the PERFUSE study. Rheumatol Adv Pract 7(2): rkad031.
- Marona J, Sepriano A, Ramiro S, Almeida D, Brites L, et al. (2023) Effectiveness of biosimilar infliximab CT-P13 compared to originator infliximab in biological-naïve patients with rheumatoid arthritis and axial spondyloarthritis: data from the Portuguese Register. Effectiveness of biosimilar infliximab CT-P13 compared to originator infliximab in biological-naïve patients with rheumatoid arthritis and axial spondyloarthritis: data from the Portuguese Register. ARP Rheumatol 2(2): 132-140.
- Bechman K, Halai K, Yates M, Norton S, Cope A, et al. (2021) Nonserious infections in patients with rheumatoid arthritis: results from the british society for rheumatology biologics register for rheumatoid arthritis. Arthritis Rheumatol 73(10): 1800-1809.
- Jørgensen KK, Olsen IC, Goll GL, Lorentzen M, Bolstad N, et al. (2017) Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. The Lancet 389(10086): 2304-2316.
- Hanauer S, Liedert B, Balser S, Brockstedt E, Moschetti V, et al. (2021) Safety and efficacy of BI 695501 versus adalimumab reference product in patients with advanced Crohn's disease (VOLTAIRE-CD): a multicentre, randomised, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol 6(10): 816-825.
© 2024 Hanan Abushwereb. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






