- Submissions

Full Text
Orthopedic Research Online Journal
The Orthopedic Burden of U.S. Cancer Care
Lauren Zeitlinger*
WellSpan Medical Education, USA
*Corresponding author: Lauren Zeitlinger, Well Span Medical Education, USA
Submission: September 19, 2017; Published: October 26, 2017

ISSN : 2576-8875Volume1 Issue2
Abstract
Cancer treatment and survivorship management continue to be rapidly evolving aspects of modern healthcare systems. As cancer survivorship has changed, the effects of prescribed treatments and their long-term morbidities are beginning to be understood, necessitating awareness by the orthopaedic profession of the diagnostic and management challenges of cancer patients with musculoskeletal complaints. The likelihood that cancer patients and cancer survivors will seek orthopedic evaluation for a consequence of treatment is reasonably high, and likely to continue to expand. We help outline the consequences of cancer treatment that warrant unique orthopedic considerations.
Introduction
As the longevity of people in the developed world increases, the incidence and prevalence of cancer continues to increase. In 2016, 1,685,210 new cancers were diagnosed in the United States, with an overall incidence of 454.8/100,000 individuals per year, and an estimated 50% of these patients may develop osseous metastatic disease [1,2]. As diagnostic and treatment strategies continue to evolve, the prevalence of cancer survivors is also increasing, with 5-year survivorship rates rising from 49% in 1975-77 to over 68% in 2003-2009 [2-4]. The number of individuals living beyond a cancer diagnosis will reach nearly 26.1 million individuals by 2024. It is estimated that roughly 39.6% of individuals will be diagnosed with some form of malignancy during their lifetime [3].
A 2014 census of the membership of the American Academy of Orthopaedic Surgeons (AAOS) estimates that Orthopaedic Surgeons in the United States performed over 9.75 million orthopaedic procedures, and several million more patient encounters for nonoperative musculoskeletal conditions [4]. This suggests a statistical inevitability that every practicing orthopaedic surgeon will be involved in the care of cancer patients and cancer survivors at some point in their career. It is critical, therefore, for orthopaedists to have an understanding of the burden that cancer and the long-term complications associated with cancer treatment place on the orthopaedic profession, so as to identify specific interventions that can optimize patient outcomes.
Primary Musculoskeletal Malignancies
Primary bone tumors
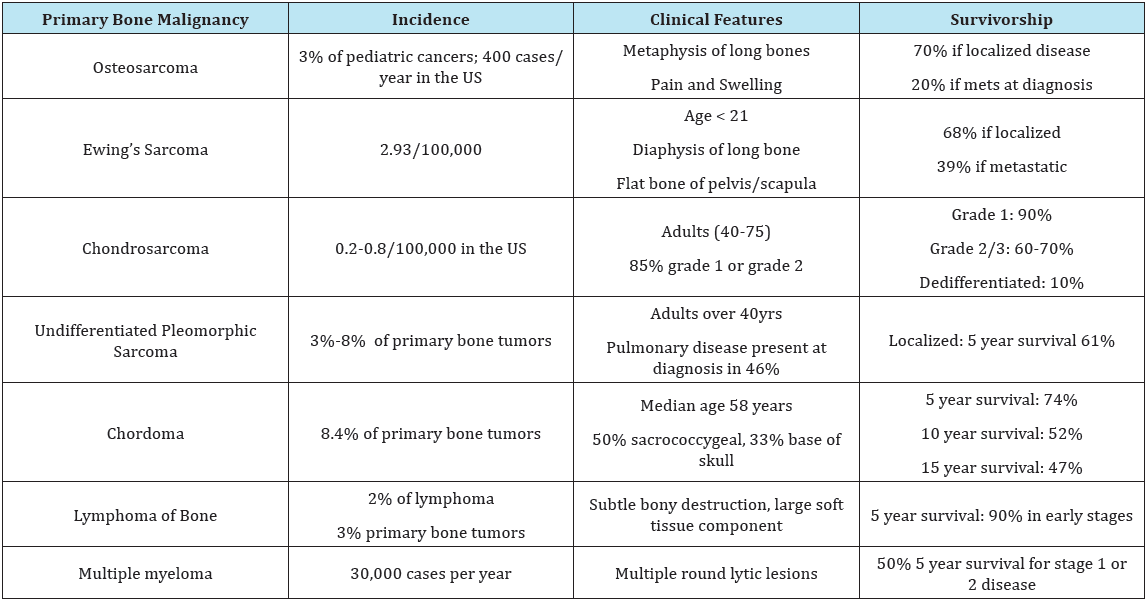
Primary bone lesions are more commonly identified in patients under the age of 40, and can be subdivided into benign and malignant lesions. Orthopedic Surgeons are expected to identify lesions with malignant characteristics that require referral to an Orthopedic Oncologist. Painful lesions, or lesions associated with other systemic signs such as fevers or unintentional weight loss warrant a thorough evaluation and possible referral to an oncologic specialist. Radiologic features of aggressiveness such as cortical destruction, expansive growth, permeative growth, or periosteal reaction require prompt referral to an Orthopedic Oncologist for biopsy and definitive treatment. Osteosarcoma, Ewing sarcoma and chondrosarcoma represent 70% of primary bone tumor diagnoses, and malignant primary bone tumors represent around 3,300 new cases annually in the United States [5,6].
Table 1: Clinical features, incidence, and survivorship of malignant primary bone tumors [5-7].
Multiple myeloma
Technically considered a plasma cell dyscrasia, multiple myeloma is the most common primary malignancy of bone [7,8]. The presence of multiple osteolytic bone lesions is a defining trademark of the disease. As many as 30% of myeloma patients may present with a pathologic fracture, and up to two-thirds with bone pain alone as the initial symptom of disease [7]. In 2016 the incidence of multiple myeloma was close to 30,000 individuals. Survivorship has increased dramatically over the past decade, and over 50% of newly diagnosed cases are expected to live 5 years or more [8] (Table 1).
Primary soft tissue tumors
Nearly 9,000 soft tissue malignancies are diagnosed in the U.S. each year [9]. It is critical for the orthopedic practitioner to have a high degree of suspicion for soft tissue sarcoma, and to evaluate and refer appropriately. A mass that is firm, deep to fascia, rapidly growing, or larger than a table tennis ball should prompt further investigation. If a biopsy is warranted, referral to a musculoskeletal oncologist should be made to ensure that the biopsy does not adversely affect definitive surgical management in the future [10].
Metastatic disease
More than 50% of patients with terminal cancers are likely to develop a bone metastasis at some point. It is therefore a certainty that all orthopedic surgeons will see this problem in their career [11-14]. The practicing orthopaedist should expect to encounter metastatic disease to bone with some frequency. Breast, prostate, lung, thyroid, and renal carcinomas are the most common primary malignancies to spread to bone, with a recent increase in rates of malignant melanoma and gastrointestinal carcinomas as advancements in immunotherapy and other modalities improve survival rates in these patients [15,16]. Table 2 illustrates the overall number, survivorship, and rates of osseous metastasis of these common malignancies.
Table 2: Disease-specific survivorship and rates of metastatic disease to bone [11-16].
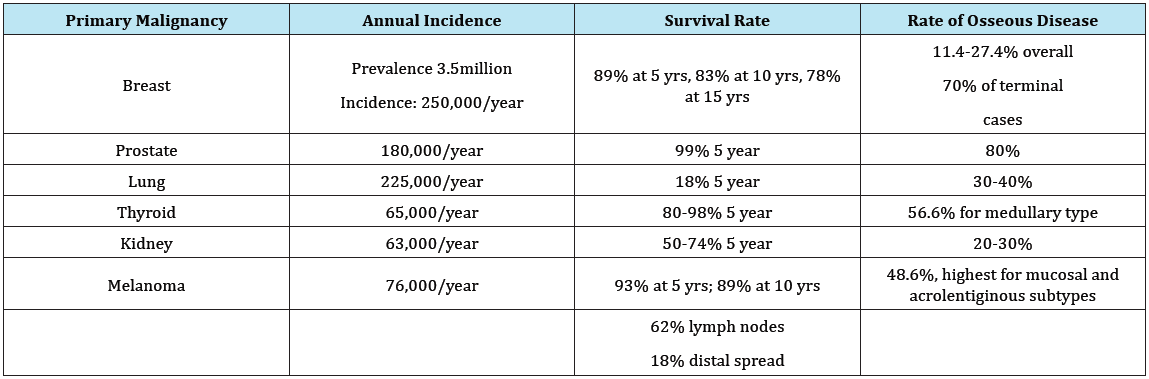
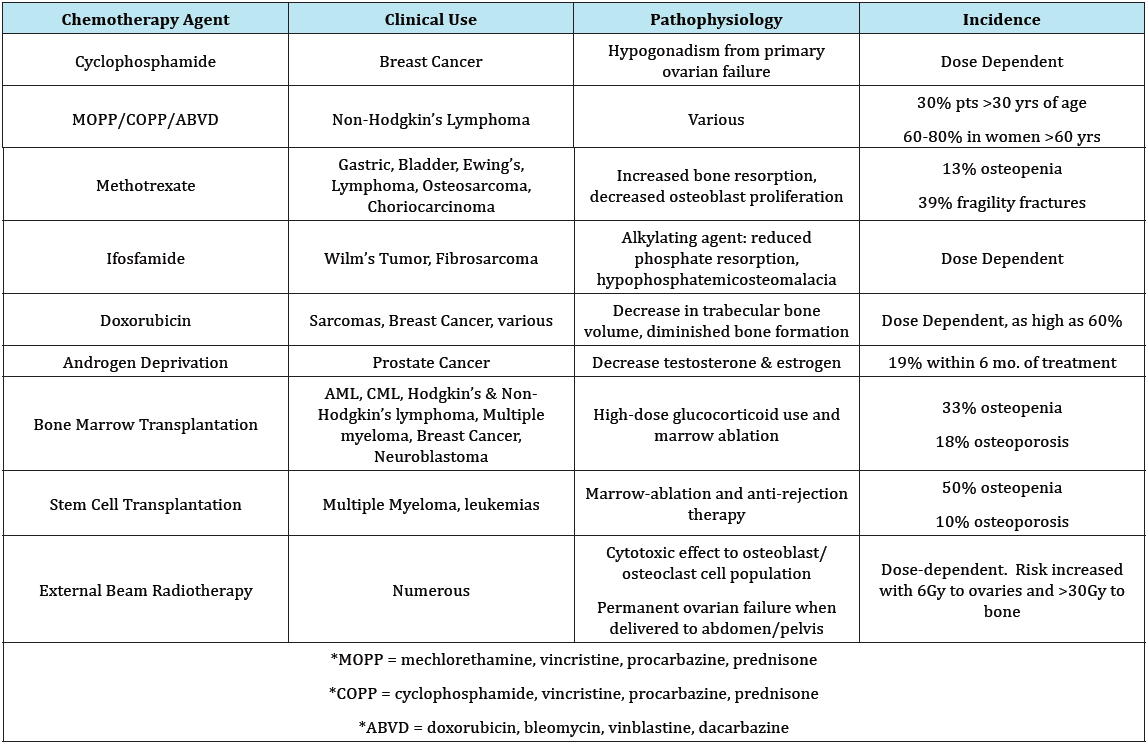
Table 3: Specific Therapy effects on bone density [24, 32-42].
Short Term Complications of Musculoskeletal Malignancy
Electrolyte abnormalities
Malignancy is the second most common cause of undiagnosed hypercalcemia. Tumor production of PTHrp, 1, 25-dihydroxyvitamin D, and stimulation of osteoclast activation induce osteolysis [17]. The clinical presentation of hypercalcemia can range from mild to life threatening. Generalized bone pain, thirst, constipation and abdominal pains, biliary and renal stone formation, mental confusion, and eventually coma can manifest from elevated calcium level. Malignancies most commonly associated with increased PTHrp production include renal, breast, ovarian, and lung carcinomas, leukemia, and some lymphomas [17] (Table 3).
An estimated 20-30% of newly diagnosed cancer patients will demonstrate hypercalcemia, and this should be considered the most urgently life-threatening complication that should prompt immediate treatment. Treatment includes rehydration, loop diuretics and IV bisphonsonates or denosumab [18]. The orthopaedist called upon to evaluate a concerning bone lesion or pathologic fracture should have a high clinical suspicion for hypercalcemia, and initiate necessary treatments immediately.
Extremity pain and lytic bone lesions
Metastatic disease to bone should be considered in any patient with a history of cancer and new onset bone pain, lowenergy fractures, fractures unusual for the mechanism of injury, or when obvious lesions are identified on radiographic imaging. The orthopaedic surgeon must be able to decide whether or not to operate on a patient with metastatic disease without pathologic fracture. Several clinical scoring systems and imaging-based structural rigidity analyses have been developed to assist the orthopaedic practitioner predict the likelihood of fracture [19-21]. The wide range of surgical treatment options is beyond the scope of this conversation, but the goals of treatment should always focus on pain relief and functional optimization. The surgical intervention planned should provide a durable construct that is expected to provide indefinite stability until the patient’s death.
Tumor-mediated osteolysis results from tumor stimulation of osteoclast precursor cells via the RANKL-RANK pathway [22]. Osteoclasts stimulate VEGF-A which directs angiogenesis and enables tumor survival, and osteolysis releases TGF-B which leads to production of PTHrp [22,23]. This self-destructive cycle of osteolysis perpetuates itself, and poses a risk for pathologic fracture that frequently necessitates orthopedic intervention. The orthopaedic surgeon is responsible for recognizing this and ensuring that patients with osseous metastases are initiated on antiresorptive therapy to block the RANK-RANKL pathway and reduce the risk of future skeletal events [24].
Spinal cord compression
Pain is the presenting complaint in 83-95% of patients with metastatic spine disease. Point tenderness, pain that occurs at rest, pain with movement due to instability, cord compression, or radicular pain should prompt investigation [25]. Degenerative thoracic pain is much less common than lumbar and cervical pain, so new onset pain in the thoracic region should raise suspicion for cancer [25]. A comprehensive neurologic exam is critical to identify neurological deficits so that early intervention can preserve function and mobility. The spine is the most common site of metastatic bone disease, observed in up to 40% of patients with bony involvement. Of these patients, 5-10% will demonstrate some degree of spinal cord compression [26].
The Management of spinal metastases includes various surgical techniques, radiation therapy, and percutaneous ablation and augmentation. While life expectancy is a common part of the treatment algorithm, longevity may be difficult to predict [27]. Strong indications for surgical intervention include spinal instability, radio resistant tumors, significant kyphotic deformity, neurologic compromise, and lesions that have failed radiotherapy [28,29]. When comparing combined corticosteroid and radiotherapy versus surgical decompression and postoperative radiotherapy for patients with cord or nerve compression from metastatic disease, surgical decompression was associated with better preservation of ambulatory function and mobility [30,31].
Long term complications of cancer treatment
As cancer survivorship increases, and modalities for treatment continue to evolve, orthopedists will increasingly be tasked with caring for the long term consequences of cancer therapies. A thorough treatment history should be obtained in any patient with a history of cancer. Specific consequences that are highly relevant to orthopedic surgeons include bone density loss, degenerative joint disease, loss of function, and an increased risk for injuries and secondary malignancies.
Bone density loss
Cancer and cancer treatments cause bone loss via several different mechanisms. Aberrant hormonal regulation from chemotherapy agents can induce early menopause and premature ovarian failure. Luteinizing hormone (LH) agonists and antiestrogen and anti-androgen therapies used in the treatment of breast and prostate cancers affect bone density in profound ways [32-34]. Estrogen deficiency has a clear relationship to bone loss in women. Women with drug-induced ovarian failure or menopause have a faster rate of bone demineralization that begins at younger ages compared to matched controls [35]. Among long-term breast cancer survivors, 34.8% develop osteopenia and 11.3% develop osteoporosis within 6 years of diagnosis [36,37].
For breast cancer survivors treated with radiation, surgery, and anastrozole, an overall mineralization decline was observed within 12 months of treatment [36]. Ovarian failure rates may be as high as 85-100% when receiving combined chemotherapy regimens including cyclophosphamide and fluorouracil with either methotrexate or doxorubicin. For breast cancer survivors who sustained a fracture, aromatase inhibitors were the most common prior therapy [24]. Hip or femur fractures accounted for 29% of fragility fractures, followed by rib, vertebral and wrist fractures [36].
Decreased estrogen and bioavailable testosterone are prime suspects for osteoporosis and fragility fractures in men. Osteoporosis due to androgen deprivation therapy in men can be as high as 53% depending on the duration of treatment [38]. Given the expected increase in insufficiency fractures over the next several years, it becomes increasingly important to consider active bone density management in patients with a history of cancer. Bone density loss is particularly important to address in survivors of pediatrics cancers, particularly females. Young women treated with radiation therapy below the diaphragm, and alkylating agents including those mentioned above, demonstrate an increased risk for early menopause at a rate nearly 4 times that of women treated with radiation alone [24,39].
The incidence of early menopause ranged from 2% of bone cancer survivors to 10% for survivors of either Hodgkin’s disease or non-Hodgkin’s lymphoma. Furthermore, the risk of menopause increase with increased age at diagnosis. Among all pediatric cancer survivors, there is a 25% rate of long-term osteopenia and/ or osteoporosis [39]. Lastly, bone density is also affected by the use of glucocorticoids [40]. Steroids are a component of several oncology regimens. Where ashormonal antagonists contribute to bone loss via the protective endocrine pathways, glucocorticoids inhibit osteoblast activity [41]. Daily doses of corticosteroid from 7.5mg to 11.5mg of prednisone equivalent can lead to osteopenia. The most rapid bone loss occurs in the first year of treatment, then an accelerated rate of loss persists thereafter. The effect of cotiscosteroids on bone density is directly related to the dose and duration of treatment [40].
Management strategies for bone density loss in cancer patients have yet to be perfected. Bisphosphonates slow the process of bone resorption that occurs with age and hormone-related bone density loss [42]. However, the side effects of treatment such as GERD, jaw necrosis, and atypical fractures preclude their long-term use. Denosumab, a monocolonal antibody against RANKL, has shown promising results with the benefit of improved tolerance compared to bisphosphonates, and greater safety for longer periods of treatment [43]. The orthopaedist must emphasize additional prevention measures including adequate calcium and vitamin D supplementation, and weight-bearing exercise [44].
Joint pain and degenerative joint disease
Long term cancer survivors will soon join the population of aging individuals seeking total joint arthroplasty. Avascular necrosis (AVN) and the subsequent need for TJA is elevated in recipients of bone marrow transplants [45,46]. The incidence of AVN after stem cell transplant is 2.9% with autologous donor, 5.4% with matched related donor and 15% after unrelated donor. In patients receiving corticosteroids for myeloma and other hematologic malignancies, AVN has been identified as a complication in roughly 9% of patients, correlated with cumulative dexamethasone dose, male sex and younger age at the time of therapy [47]. Roughly 80% of patients with femoral head AVN will require hip replacement within 5 years of diagnosis [48].
For survivors of pediatric leukemia and lymphoma, the overall incidence of AVN is 7.6%, diagnosed at an average of 17 months after treatment [49]. Younger patients with treatment-related avascular necrosis generally seek total joint arthroplasty within 20 years of diagnosis [48]. It is important to take a cancer patient’s history into consideration when planning for any joint replacement, as the risks for perioperative complications in cancer patients may be substantially different from that of the general population. Active malignancy is associated with an increased risk of infection and thromboembolism, as cancer patients are hyper coagulable at baseline [50]. In the elderly population, the presence of metastatic cancer is associated with an increased 90 day mortality in total hip arthroplasty. Therefore, patient selection and careful management is critical [51].
Loss of function
Loss of function and impaired mobility are common side effects from cancer and its related therapies. In 2013, it was reported that of 13.8 million cancer survivors, 20% of children and 53% of adults had difficulty with physical function, either from treatment or cancer itself [52]. In a large survey of cancer survivors, 31% of survivors reported some persistent adverse health affect, and younger patients most commonly reported arthritis and osteoporosis [53]. Upper extremity pain and dysfunction is increasingly common in breast cancer survivors. Up to one third of breast cancer survivors report extremity circulation problems [54]. Not including postsurgical pain, patients treated for breast cancer with mastectomy, axillary dissection and/or adjuvant radiotherapy often suffer from adhesive capsulitis, mononeuropathy, rotator cuff disease, lymphedema, radiation osteiitis, and axillary web syndrome [55]. When comparing range of motion and strength between women who had undergone mastectomy versus mastectomy and radiation, 40% of women reported impairment of shoulder function when radiated, 31% for surgery alone.
There was a significant reduction of range of motion in all planes in irradiated shoulders, and reduction in flexion only with surgery alone. Additionally, strength was found to be diminished on the operative side, worse when combined with radiation therapy [56,57]. In addition to upper extremity complications, postural effects commonly ensue, and postural failures are seen in 83% of breast cancer survivors as a result of treatment [58]. Surgery, chemotherapy, and radiation can all contribute to post-treament neuropathy. Chemotherapy-induced neuropathy is a common adverse effect of treatment, and reliable methods to prevent and treat neurotoxicity have not been identified [59]. Sensory alterations are the most common neuropathy.
More severe toxicity leads to pain, burning, and aching sensations that become limiting, this may cause ataxia and gait disturbance in the lower extremities, or clumsiness and diminished dexterity in the upper extremities. Treatment is largely symptomatic control with duloxetine, anticonvulsants, tricyclic antidepressants, and topical anesthetics, although the data is far from conclusive [59]. Radiation effects may be immediately apparent during the course of treatment, including burns or wounds, or may not become apparent for many years after treatment and evolve into fibrosis, joint contractures, and secondary malignancies. Radiation fibrosis is a major contributor to long term disability, and may mimic adhesive capsulitis, rotator cuff pathology, or brachial plexopathy [55,60]. Radiation-associated neuropathy is much less predictable than chemotherapy-induced neuropathy [61].
Brachial plexus neuropathy is increasingly common in breast cancer survivors, and the pathophysiology is thought to be due to microvascular damage combined with long term constrictive fibrosis. The development of radiation-induced neuropathy is directly related to dose delivered. A total dose between 34-40Gy is associated with a neuropathy risk less than 1%, but doses between 43.5-60Gy increase risk to 73%. High fractional doses further contribute to the risk of plexopathy [61]. For head and neck cancers treated with radiation, 14% of patients studied reported symptoms consistent with brachial plexus neuritis [62].
Lymphedema is a chronic condition characterized by retention of interstitial fluid due to impaired lymphatic drainage and venous return. It can contribute to increased limb weight, paresthesias, stiffness, diminished range of motion, and additional emotional difficulties. The overall incidence of lymphedema in the upper extremities of patients with breast cancer is as high as 65% [63,64]. The etiology of lymphedema is multi factorial, from any combination of surgical lymph node dissection and radiotherapy [64,65]. Although incurable, evolving modalities in physiotherapy and manual lymph drainage have been effective in symptom management, and early referral to lymphedema clinics is crucial for cancer survivors [65].
Conclusion
Cancer incidence and cancer survivorship numbers are growing in the United States and worldwide. In addition to the immediate orthopaedic complications of active malignancy, the orthopaedic practitioner should be aware of long-term complications that cancer survivors may manifest. A thorough inventory of the patient’s prior diagnoses and treatments will enable the orthopaedic surgeon to properly assess the patient’s risk of fragility fractures, joint degeneration, perioperative complications, and likelihood of success prior to surgery or other treatments.
The orthopaedic community must also take an active role in the management of bone density for cancer survivors who may present with complications of osteopenia/osteoporosis several years before routine densitometry is recommended by primary care providers. Any patient with a history of cancer who presents with new complaints of pain, swelling, or a mass should be evaluated more comprehensively. The burden that cancer care places on the orthopaedic community will increase sharply in the coming year, and the practicing orthopaedist must be equipped to handle the complexities of these patients.
References
- American Cancer Society (2006) Cancer Treatment & Survivorship Facts & Figures 2016-2017. American Cancer Society, Atlanta, USA.
- Bluethmann SM, Mariotto AB, Rowland JH (2016) Anticipating the ‘’Silver Tsunami’’: Prevalence Trajectories and Comorbidity Burden among Older Cancer Survivors in the United States. Cancer Epidemiol Biomarkers Prev 25(7): 1029-1036.
- Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, et al. (2017) Annual Report to the Nation on the Status of Cancer, 1975-2014, Featuring Survival. J Natl Cancer Inst 109 (9).
- American Academy of Orthopaedic Surgeons (2015) Orthopaedic Practice in the U.S. 2014. AAOS Department of Research and Scientific Affairs, USA.
- Natia E, Goodman M, Marcus RB (2008) Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: Surveillance Epidemiology and End Results data. J Pediatr Hematol Oncol 30(6): 425- 430.
- Lee FY, Mankin HJ, Fondren G, Gebhardt MC, Springfield DS, et al. (1999) Chondrosarcoma of bone: an assessment of outcome. J of Bone Joint Surg Am 81(3): 326-338.
- Scharschmidt TJ, Thomas JD, Becker PS, Conrad EU (2011) Multiple myeloma: diagnosis and orthopaedic implications. Journal of the American Academy of Orthopaedic Surgeons 19(7): 410-419.
- Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, et al. (2008) Improved survival in multiple myeloma and the impact of novel therapies. Blood 2008; 111(5): 2516-2520.
- Clark MA, Fisher C, Judson I, Thomas JM (2005) Soft-tissue sarcomas in adults. New Engl J Med 353(7): 701-711.
- Qureshi YA, Huddy JR, Miller JD, Strauss DC, Thomas JM, et al. (2012) Unplanned excision of soft tissue sarcoma results in increased rates of local recurrence despite full further on cological treatment. Ann Surg Oncol 19(3): 871-877.
- DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, et al. (2014) Cancer treatment and survivorship statistics, 2014. CA a cancer J clin 64(4): 252-271.
- Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, et al. (2010) Metastatic behavior of breast cancer subtypes. J clin oncol 28(20): 3271- 3277.
- Mirallie E, Vuillez JP, Bardet S, Frampas E, Dupas B, et al. (2005) High frequency of bone/bone marrow involvement in advanced medullary thyroid cancer. J Clin Endocrinol Metab 90(2): 779-788.
- Selvaggi GG Scagliotti V (2005) Management of bone metastases in cancer: a review. Critical reviews in oncology/hematology 56(3): 365- 378.
- Sim FH, Nelson TE, Pritchard DJ (1997) Malignant melanoma: Mayo Clinic experience. Mayo Clinic Proceedings 72(6): 565-559.
- Zekri J, Ahmed N, Coleman RE, Hancock BW (2001) The skeletal metastatic complications of renal cell carcinoma. Intl J oncol 19(2): 379- 382.
- Clines GA, Guise TA (2005) Hypercalcemia of malignancy and basic research on mechanisms responsible for osteolytic and osteoblastic metastasis to bone. Endoc Relat Cancer 12(3): 549-583.
- Mirrakhimov AE (2015) Hypercalcemia of malignancy: an update on pathogenesis and management. N Am J Med Sci 7(11): 483.
- Mirel H (1989) Metastatic disease in long bones. A proposed scoring systemic for diagnosing impending fractures. Clin Orthop Relat Res 249: 256-264.
- Damron TA, Nazarian A, Entezari V, Brown C, Grant W, et al. (2016) CTbased structural rigidity analysis is more accurate than Mirels scoring for fracture prediction in metastatic femoral lesions. Clin Orthop Relat Res 474 (3): 643-651.
- Van Der Linden YM, Dijkstra PD, Kroon HM, Lok JJ, Noordijk EM, et al. (2004) Comparative analysis of risk factors for pathological fractures with femoral metastasis. J Bone Joint Surg Br 86 (4): 566-573.
- Lynch CC (2011) Matrix Metalloproteinases as master regulatory of the viscious cycle of bone metastasis. Bone 48(1): 44-53.
- Quinn JM, Matsumura Y, Tarin D, McGee JO, Athanasou NA (1994) Cellular and hormonal mechanisms associated with malignant bone resorption. Laboratory investigation; a journal of technical methods and pathology 71(4): 465-471.
- Rizzoli R, Body JJ, Brandi ML, Cannata-Andia J, Chappard D, et al. (2013) Cancer-associated bone disease. Osteoporos Int 24(12): 2929-2953.
- Quraishi NA, Claire E (2011) Metastatic spinal cord compression. BMJ: British Medical Journal 342.
- Schmidt MH, Paul K, Frank DV (2005) Metastatic spinal cord compression. Journal of the National Comprehensive Cancer Network 3(5): 711-719.
- Nathan SS, Healey JH, Mellano D, Hoang B, Lewis I, et al. (2005) Survival in patients operated on for pathologic fracture: Implications for end-oflife orthopedic care. J Clin Oncol 23(25): 6072-6082.
- Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, et al. (2001) Surgical Strategy for spinal metastases. Spine 26(3):298-306.
- Tokuhasi Y, Matsuzaki H, Oda H, Oshima M, Ryu J (2005) A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine 30(19): 2186-2191.
- Ghogawala Z, Mansfield FL, Borges LF (2001) Spinal radiation before surgical decompression adversely affects outcomes of surgery for symptomatic metastatic spinal cord compression. Spine 26(7): 818- 824.
- Patchell RA, Tibbs PA, Regine WF, Payne R, Saris S, et al. (2005) Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. The Lancet 366(9486): 643-648.
- Peters CA, Walsh PC (1987) The effect of nafarelin acetate, a luteinizinghormone- releasing hormone agonist, on benign prostatic hyperplasia. New England Journal of Medicine 317(10): 599-604.
- Love RR, Mazess RB, Barden HS, Epstein S, Newcomb PA, et al. (1992) Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. New England Journal of Medicine 326(13): 852-856.
- Smith MR, McGovern FJ, Zietman AL, Fallon MA, Hayden DL, et al. (2001) Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. New England Journal of Medicine 345(13): 948-955.
- Reid DM, Doughty J, Eastell R, Heys SD, Howell A, et al. (2008) Guidance for the management of breast cancer treatment-induced bone loss: a consensus position statement from a UK Expert Group. Cancer treatment reviews 34(suppl 1): S3-S18.
- Edwards BJ, Raisch DW, Shankaran V, McKoy JM, Gradishar W, et al. (2011) Cancer therapy associated bone loss: implications for hip fractures in mid-life women with breast cancer. Clinical Cancer Research 17(3): 560-568.
- Abdel-Razeq H, Abdulla A (2011) Bone health in breast cancer survivors. Journal of cancer research and therapeutics 7(3): 256-263.
- Lassemillante AC, Doi SA, Hooper JD, Prins JB, Wright OR, et al.(2014) Prevalence of osteoporosis in prostate cancer survivors: a meta-analysis. Endocrine 45(3): 370-381.
- Chiarelll AM, Marrett LD, Gerarda D (1999) Early menopause and infertility in females after treatment for childhood cancer diagnosed in 1964–1988 in Ontario, Canada. American Journal of Epidemiology 150(3): 245-254.
- Reid IR (1997) Glucocorticoid osteoporosis: Mechanisms and management. Eur J Endocrinol 137(3): 209-217.
- Pfeilschifter J, Diel IJ (2000) Osteoporosis due to cancer treatment: pathogenesis and management. J Clin Oncol 18(7): 1570-1593.
- Lustberg MB, Reinbolt RE, Shapiro CL (2012) Bone health in adult cancer survivorship. J Clin Oncol 30(30): 3665-3674.
- Lee BL, Higgins MJ, Goss PE (2012) Denosumab and the current status of bone-modifying drugs in breast cancer. Acta Oncol 51(2): 157-167.
- Saarto T, Sievänen H, Kellokumpu-Lehtinen P, Nikander R, Vehmanen L, et al. (2012) Effect of supervised and home exercise training on bone mineral density among breast cancer patients. A 12-month randomised controlled trial. Osteoporos Int 23(5): 1601-1612.
- Socié G, Salooja N, Cohen A, Rovelli A, Carreras E, et al. (2003) Nonmalignant late effects after allogeneic stem cell transplantation. Blood 101(9): 3373-3385.
- Bizot P, Witvoet J, Sedel L (1996) Avascular necrosis of the femoral head after allogenic bone-marrow transplantation-a retrospective study of 27 consecutive THAs with a minimal two year follow up. J Bone Joint Surg 78-B(6): 878-883.
- Campbell S, Sun CL, Kurian S, Francisco L, Carter A, et al. (2009) Predictors of avascular necrosis in bone in long-term survivors of hematopoietic cell transplantation. Cancer 115(18): 4127-4135.
- Niinimäki R, Hansen LM, Niinimäki T, Olsen JH, Pokka T, et al.(2013) Incidence of severe osteonecrosis requiring total joint arthroplasty in children and young adults treated for leukemia or lymphoma: a nationwide, register-based study in Finland and Denmark. J Adolesc young adult oncol 2(4): 138-144.
- Salem KH, Brockert AK, Mertens R, Drescher W (2013) Avascular necrosis after chemotherapy for haematological malignancy in childhood. Bone Joint J 95-B(12): 1708-1713.
- Everhart JS, Altneu E, Calhoun JH (2013) Medical comorbidities are independent preoperative risk factors for surgical infection after total joint arthroplasty Clin Orthop Relat Res 471(10): 3112-3119.
- Bozic KJ, Lau E, Kurtz S, Ong K, Rubash H, et al. (2012) Patient-related risk factors for periprosthetic joint infection and postoperative mortality following total hip arthroplasty in Medicare patients. The Journal of Bone & Joint Surgery 94(9): 794-800.
- Stubblefield MD, Schmitz KH, Ness KK (2013) Physical functioning and rehabilitation for the cancer survivor. Semin oncol 40(6): 784-795.
- Schultz PN, Beck ML, Stava C, Vassilopoulou-Sellin R (2003) Health profiles in 5836 long-term cancer survivors. Int J Cancer 104(4): 488- 495.
- Stava C, Todd Weiss L, Vassilopoulou-Sellin R (2006) Health Profiles of 814 very long term breast cancer survivors. Clin Breast Cancer 7(3): 228-236.
- Stubblefield MD, Keole N (2014) Upper body pain and functional disorders in patients with breast cancer. PM R 6(2): 170-183.
- Haddad CA, Siaãd M, Perez Mdel C, Miranda Júnior F (2013) Assessment of posture and joint movements of the upper limbs of patients after mastectomy and lymphadenectomy. Einstein (Sao Paulo) 11(4): 426- 434.
- Blomquivist L, Stark B, Natacha E, Malm M (2004) Evaluation of arm and shoulder mobility and strength after modified radical mastectomy and radioterapy. Acta Oncol 43(3): 280-283.
- Malicka I, Barczyk K, Hanuszkiewicz J, Skolimowska B, Wozniewski M (2010) Body posture of women after breast cancer treatment. Ortop Traumatol Rehabil 12(4): 353-361.
- Grisold W, Guido C, Windebank AJ (2012) Peripheral neuropathies from chemotherapeutics and targeted agents: diagnosis, treatment, and prevention. Neuro-oncology 14(suppl 4): iv45-iv54.
- Stubblefield MD (2011) Radiation fibrosis syndrome: neuromuscular and musculoskeletal complications in cancer survivors. PM R 3(11): 1041-1054.
- Powell S, Cooke J, Parsons C (1990) Radiation induced brachial plexus injury: follow-up of two different fractionation schedules. Radiother Oncol 18(3): 213-220.
- Chen, Allen M, Wang PC, Daly ME, Cui J, Hall WH, et al.(2014) Dosevolume modeling of brachial plexus-associated neuropathy after radiation therapy for head-and-neck cancer: findings from a prospective screening protocol. Int J Radiat Oncol Biol Phys 88(4): 771-777.
- Hayes SC, Janda M, Cornish B, Battistutta D, Newman B (2008) Lymphedema after breast cancer: incidence, risk factors, and effect on upper body function. Journal of Clinical Oncology 26(21): 3536-3542.
- Paskett ED, Dean JA, Oliveri JM, Harrop JP (2012) Cancer-related lymphedema risk factors, diagnosis, treatment, and impact: a review. Journal of Clinical Oncology 30(30): 3726-3733.
- Warren LE, Miller CL, Horick N, Skolny MN, Jammallo LS, et al. (2014) The impact of radiation therapy on the risk of lymphedema after treatment for breast cancer: a prospective cohort study. Int J Radiat Oncol Biol Phys 88(3): 565-571.
© 2017 Lauren Zeitlinger . This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






