- Submissions

Full Text
Orthopedic Research Online Journal
Pharmacologic Venous Thromboembolism Prophylaxis in Total Knee and Hip Arthroplasty: A Literature Review
Josh Hockett* and Charles Orth#
*Corresponding author: Josh Hockett, St. Mary’s Medical Center, Blue Springs, MO, USA
Submission: August 08, 2017; Published: August 21, 2017
Secondary author: Charles Orth, DO, FAOAO

ISSN : 2576-8875Volume1 Issue1
Abstract
Venous thromboembolism (VTE) is a potentially devastating and somewhat common complication following total knee and total hip arthroplasty. Typical post-operative VTE prophylaxis consist of combination mechanical and pharmacologic methods. Recommendations and clinical practices vary on choice of pharmacologic agent used for VTE prophylaxis. Many studies have been performed attempting to display superiority of certain VTE prophylaxis agents, but considerable debate still exists.
Keywords: Post-operative VTE; pharmacologic VTE prophylaxis; Total hip arthroplasty; Total knee arthroplasty; Venous thromboembolism; Deep venous thrombosis; Pulmonary embolus
Introduction
Much variability exists in post-operative VTE pharmacologic prophylaxis following total knee and total hip arthroplasty. Much literature has been published attempting to shed light on this subject, but many questions remain. What is the best pharmacologic VTE prophylaxis agent? What defines best? Least cases of symptomatic deep venous thrombosis (DVT) or pulmonary embolus (PE)? Least number of adverse side effects/bleeding? The goal of this article is to review the latest, most relevant literature regarding different pharmacologic agents used for post-operative VTE prophylaxis following total knee and hip arthroplasty. Hopefully this article will provide the reader with appropriate evidence to confidently implement a safe, effective, and patient-specific post-operative VTE prophylaxis regimen in their own practice.
Review of Literature
Many pharmacologic agents are used for VTE prophylaxis, but the focus of this article will be placed on the more commonly used ones. According to an article published in Journal of Arthroplasty in 2012 where 634 US orthopedic surgeons were surveyed in 2008, low-molecular-weight heparin (LMWH) was most widely used agent, followed by warfarin and aspirin [1]. Since then, VTE prophylaxis prescribing tends have changed greatly. A recent Level III, therapeutic study published in Clinical Orthopedic Related Research (CORR) in 2017, where Humana and Medicare databases were queried and common VTE prophylaxis agents were compared against each other following primary TKA found that utilization of Factor Xa inhibitors and aspirin had grown considerably [2]. Over the study period of 2007 to 2015, factor Xa inhibitors utilization had compound annual growth rate of 43%, followed by aspirin at 30%, enoxaparin at 3%, and lastly warfarin at -3% [2].
The majority of literature regarding VTE prophylactic pharmacologic agents compares only 2 agents head-to-head and many display somewhat variable outcomes. A retrospective single institution study published in CORR in 2014, compared aspirin 325mg BID versus warfarin for VTE prophylaxis in knee or hip arthroplasty patients between 2000 and 2012 [3]. This study found that overall symptomatic PE rate was lower in the aspirin group compared to warfarin group, 0.14% and 1.07% respectively, also with less symptomatic DVTs, wound-related problems, and shorter hospital stays in the aspirin group [3]. Another retrospective study published in Journal of Arthroplasty in 2016 comparing aspirin to warfarin in higher risk VTE patients found that the postoperative 90-day VTE incidence, periprosthetic joint infection incidence, and mortality were all higher in the warfarin group compared to the aspirin group [4]. A prospective study published in Journal of Arthroplasty in 2012 compared aspirin to warfarin, where PE risk stratification was used per AAOS guidelines resulting in 152 standard-risk patients receiving aspirin and a comparator group of 415 patients that received warfarin without PE risk stratification [5]. Results displayed a higher rate of symptomatic PE and DVT in the aspirin group (4.6%, 7.9% respectively) compared to the warfarin group (0.7%, 1.2% respectively).
Using oral factor Xa inhibitors for VTE prophylaxis following total hip or knee arthroplasty has gained much popularity recently, in large part due to the RECORD and ADVANCE trials. The RECORD1 through RECORD4 trials were rather large double blind, randomized control trials displaying superiority of rivaroxaban over enoxaparin [6-9]. The RECORD trials found a statistically significant absolute risk reduction of the occurrence of any DVT, nonfatal PE, death, and major VTE in the rivaroxaban group for THA and TKA patients, with a not statistically significant, minimal increase in major bleeding events [6-9]. The ADVANCE trials showed similar results to the RECORD trials except comparing apixaban to enoxaparin. ADVANCE1 trial failed to show superiority of apixaban to enoxaparin with regards to occurrence of any DVT, nonfatal PE, death, and major VTE, but showed statistically significant lower rate of major bleeding events [10]. ADVANCE1 trial employed standard apixaban dosing of 2.5mg PO BID versus enoxaparin 30mg subcutaneously BID, whereas similar to the RECORD trials, ADVANCE2 and ADVANCE3 trials utilized enoxaparin 40 mg subcutaneously daily dosing [10-12]. ADVANCE2 and ADVANCE3 trials displayed superiority of apixaban to enoxaparin showing a statistically significant absolute risk reduction of the occurrence of any DVT, nonfatal PE, death, and major VTE, with a small but not statistically significant decrease in major bleeding events [11,12]. One of the main concerns with factor Xa inhibitors is rather or not they increase incidence of significant bleeding events. According to the RECORD and ADVANCE trials, there was no statistically significant increase in major bleeding events [6-12]. A meta-analysis published in the Journal of Arthroplasty in 2017, where many randomized trials up to 2016 were reviewed and results of many new oral anticoagulants were compared to results using the most common dosing of enoxaparin (40 mg subcutaneously daily) [13]. Findings from this meta-analysis displayed much lower relative risk (RR) of VTE with factor Xa inhibitors (edoxaban RR 0.49, rivaroxaban RR 0.55) compared to enoxaparin [13]. The relative risk (RR) of major bleeding was lower for apixaban (RR 0.84) compared to enoxaparin, but higher for rivaroxaban (RR 1.27) compared to enoxaparin [13]. A recent Level III, therapeutic study, referred to earlier in the paper, published in Clinical Orthopedic Related Research (CORR) in 2017, where Humana and Medicare databases were queried and common VTE prophylaxis agents were compared against each other following primary TKA [2]. Results from this study displayed a statistically significant difference in incidence of DVT at 90 days post operatively with factor Xa inhibitors having lowest incidence (2.9%), followed by aspirin (3.0%), enoxaparin (3.5%), and lastly warfarin (4.8%). Also, a statistically significant difference in incidence of PE at 90 days was found with the lowest being factor Xa inhibitors (0.9%), followed by enoxaparin (1.1%), aspirin (1.2%), and warfarin (1.6%). No statistically significant difference was found between the groups regarding incidence of major bleeding events [2].
Discussion
Many pharmacologic VTE prophylactic agents are available for use, but it definitely seems that the most common agents found in recent literature are factor Xa inhibitors, aspirin, enoxaparin, and Coumadin. All of these agents attempt to prevent the occurrence of VTE’s following TKA and THA with hopefully minimal side effects, but they all act through different mechanisms of action. The following Figure 1 provides a good summary of the different mechanism of action displayed in the coagulation cascade.
Figure 1: Summary of different anticoagulation/antiplatelet agents and mechanism of action displayed in coagulation cascade [14].
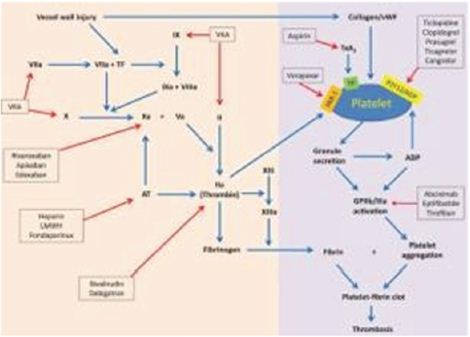
As previously mentioned, the usage of factor Xa inhibitors and aspirin has grown significantly over the recent years, likely due to emerging research [2]. The following table is from a study mentioned previously, a level III, therapeutic study published in CORR in 2017. It effectively displays the trends in utilizing the compared agents since 2007. Notice how the factor Xa inhibitors saw a large rise in utilization likely corresponding to when the favorable results of the RECORD and ADVANCE trials were published. Of course, this table only corresponds to the referenced study, but likely it can be assumed that this could be extrapolated to correspond with the trends from 2007 to 2015 of the majority of practicing US orthopedic surgeons.
Although many of the studies reviewed above, tended to display one pharmacologic agent in a better light than another, it’s rather easy to find a contradictory article. At this point, there doesn’t seem to be enough literature to state a clear winner in regards to effectiveness with best safety profile. There seems to be multiple articles reviewed above that have concluded factor Xa inhibitors are most effective at preventing postoperative VTE’s, but also conflicting evidence on whether they have increased major bleeding events [2,6-13]. There also appears to be good evidence supporting aspirin as an effective choice with a good safety profile, but questions arise on whether or not it can be used for patients with high VTE risk [2-5]. Continued research needs to be performed to determine whether one agent is superior to the others, with respect to effectiveness of preventing VTE’s while having minimal bleeding risks, regardless of the patient’s VTE risk (Table 1).
Table 1: Utilization of each agent used in the referenced study from 2007 to 2015 [2].
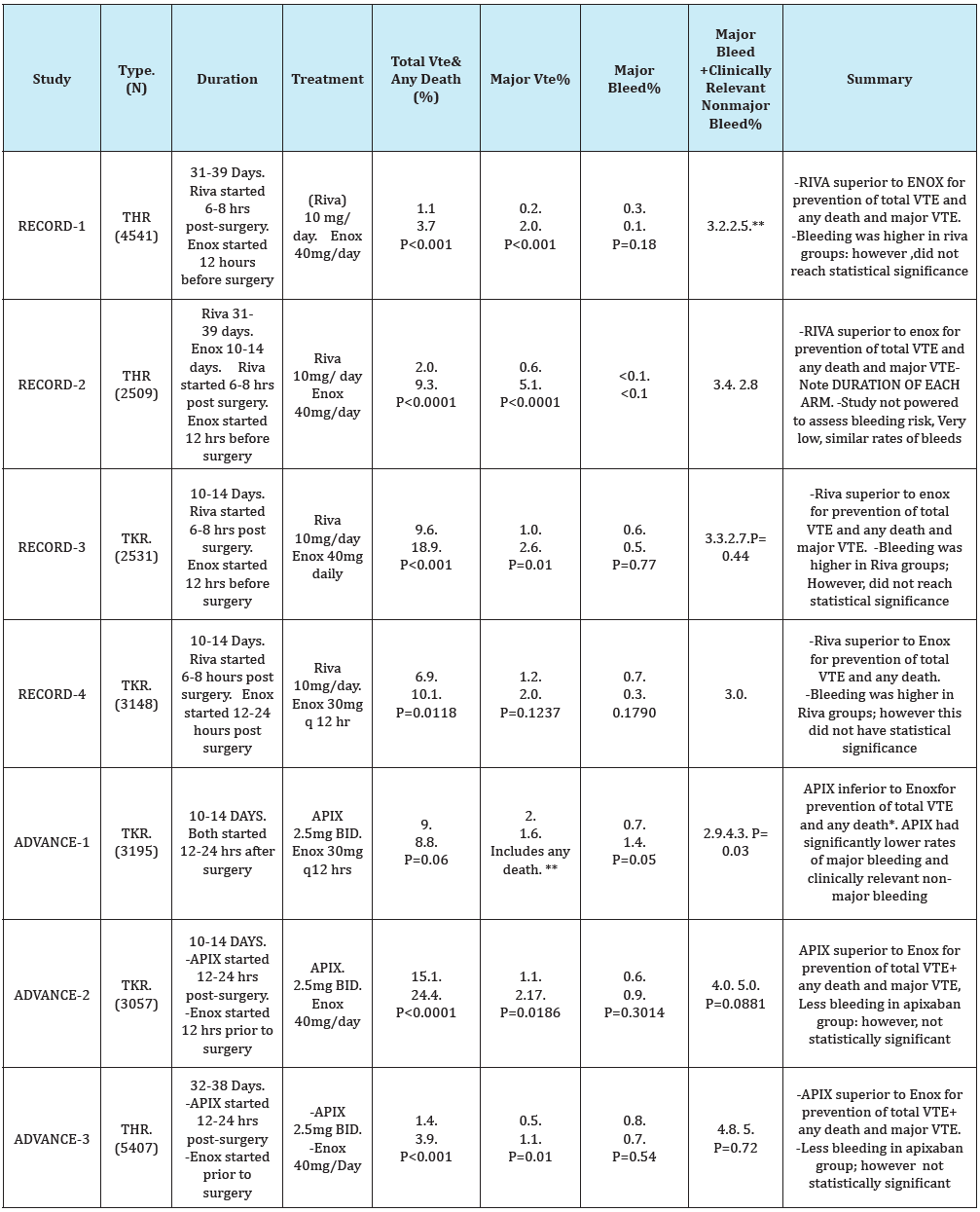
Current head to head studies comparing rivaroxaban to apixaban in current studies are lacking. Current studies available compare these two agents to the standard of therapy, enoxaparin, utilizing dosing of either 40 mg daily or 30mg twice daily. A literature review was performed to compare apixaban and rivaroxaban for venous thromboembolism prophylaxis in post-operative orthopedic total knee and total hip replacement.
For Graph Below: Abbreviations as follows
VTE=Venous thromboembolism-TKR=Total Knee Replacement- THR=Total Hip Replacement
Enox=Enoxaparin: Riva= Rivaroxaban: Apix=Apixaban
*Lower rate of VTE in enoxaparin arm of study (8.8%) compared to earlier studies (16%) meant the trial ended up being underpowered to prove non-inferiority of apixaban. Rates of primary outcome were clinically similar in enoxaparin and apixaban groups [6].
**P value not specified in study publication.
A meta-analysis including the above Phase 3 trials of apixaban and rivaroxaban pooled data demonstrated that rivaroxaban was superior to enoxaparin (p=0.0001), and apixaban had a trend toward decreased DVT,PE, and death(p=0.06) [9]. In regards to major bleeding, there was a trend toward increased risk in the rivaroxaban group (p=0.09), but not with the apixaban group(p=0.33). Apixaban had a trend toward fewer events of major bleeding plus clinically relevant non-major bleeding (p=0.06) as compared to enoxaparin; however there were more of these events in patients taking rivaroxban (p=0.02) [9].
Another large meta-analysis was conducted utilizing 16 randomized controlled trials of 38,747 patients who received rivaroxaban, apixaban, and dabigatran compared with enoxaparin from prophylaxis against VTE after total hip and total knee replacement [10]. Compared with enoxaparin , the risk of symptomatic VTE was lower with rivaroxaban but similar with dabigatran and apixaban. Compared with enoxaparin, clinically significant bleeding was higher with rivaroxaban, similar with dabigatran, and lower with apixaban [10].
Current guidelines published by the American College of Chest Physicians(ACCP), recommend the use of low-molecular weight heparin over other anticoagulant agents (Grade 2C/2B); however for patients who decline injections, ACCP does recommend the utilization of apixaban or dabigatran (Grade1B) [1]. Currently, rivaroxaban and apixaban are FDA approved for postoperative DVT thromboprophylaxis for hip and knee replacements at 10mg once daily and 2.5mg BID, respectively. ACCP recommends continued antithrombotic prophylaxis for a minimum of 10-14 days from the day of surgery for both of these indications (Grade1B). Further, for patients undergoing major orthopedic surgery, ACCP recommends extending thromboprophylaxis for up to 35 days (Grade2B) [1].
Conclusion
Venous thromboembolism is a serious, potentially fatal complication following total hip and total knee arthroplasty. Many pharmacologic VTE prophylaxis agents are used in attempts to prevent its occurrence. Much literature is in circulation advocating certain agents over others. Factor Xa inhibitors and aspirin have seemed to gain much popularity for VTE prophylaxis in the recent years, likely for good reason. Much literature has shown factor Xa inhibitors to provide better VTE prophylaxis, with no increased bleeding risk, or possibly even less bleeding risk when considering apixaban. Some literature challenges this thought, showing a potentially increased bleeding risk with factor Xa inhibitors. Aspirin has also been shown to be effective VTE prophylaxis with low bleeding risk, but can it be used with high risk VTE population? Although more research needs to be performed to determine the ideal agent, hopefully this literature review can provide the reader knowledge to confidently implement effective pharmacologic VTE prophylaxis following TKA and THA in their own practice.
References
- Falck-Ytter Y, Frances CW, Johansson NA, Curley C, Dahl OE, et al. (2012) Prevention of VTE in orthopedic surgery patients. Chest 141(2 Suppl): e278S-e325S.
- Ericsson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, et al. (2008) Study Group Rivaroxaban versus enoxaparin for thromboprophylaxisd after hip arthroplasty. N Engl J Med 358(26): 2765-2775.
- Kakkar AK, Brennan B, Dahl OE, Erickson BI, Mouret P, et al. (2008) Rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthhroplasty: a double blind, randomized controlled trial. Lancet 372(9632): 31-39.
- Lassen MR, Ageno W, Borris LC, Lieberman JR, Rosencher N, et al. (2008) Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 358(26): 2776-2786.
- Turpie AGG, Lassen MR, Davidson BL, Bauer KA, Gent M, et al. (2009) Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): A randomized trial. Lancet 373(9676): 1673- 1680.
- Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, et al. (2009) Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med 361(6): 594-604.
- Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, et al. (2010) Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomized double-blind trial. Lancet 375(9717): 807- 815.
- Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, et al. (2010) Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med 363: 2487-2498.
- Russell RD, Huo MH (2013) Apixaban and rivaroxaban decrease deep vein thrombosis but not other complications after total hip and total knee arthroplasty. J Arthoplasty 28(9): 1477-1481.
- Antonio G, Ana Isabel T, Luisa SM, Emilio V (2012) Dabigatran, rivaroxaban, or apixaban versus enoxaparin for thromboprophylaxis after total hip or knee replacement: systematic review, meta-analysis, and indirect treatment comparisons. BMJ 344: e3675.
- Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, et al. (2010) Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet 375(9717): 807- 815.
- Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, et al. (2010) Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med 363(26): 2487-2498.
- Venker BT, Ganti BR, Lin H, Lee ED, Nunley RM, et al. (2017) Safety and efficacy of new anticoagulants for the prevention of venous thromboembolism after hip and knee arthroplasty: a meta-analysis. J Arthroplasty 32(2): 645-652.
- Hess C, Norgren L, Ansel G, Capell WH, Fletcher JP, et al. (2017) A Structured Review of Antithrombotic Therapy in Peripheral Artery Disease With a Focus on Revascularization. Circulation 135(25): 2534- 2555.
© 2017 Josh Hockett, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






