- Submissions

Full Text
Open Journal of Cardiology & Heart Diseases
Heterotopic Edwards Sapien 3 Transcatheter Implantation in a Degenerated Mitral Bioprosthesis: A Case Report
Castiello DS*, Angellotti D, Simonetti F, Franzone A, Piccolo R and Esposito G
Department of Advanced Biomedical Sciences, University of Naples Federico II, Italy
*Corresponding author: Castiello DS, Department of Advanced Biomedical Sciences, University of Naples Federico II, Italy
Submission: January 27, 2023;Published: February 21, 2023

ISSN 2578-0204Volume4 Issue2
Rational
In recent years, bioprostheses are increasingly used in preference to mechanical valves for the well-known advantages. However, the occurrence of structural degeneration, limiting valve durability, remains a critical issue for bioprostheses implantation. In current definition of bioprosthetic valve dysfunction, there are four pathological categories: Structural Valve Deterioration (SVD), Non-Structural Valve Deterioration (NVD), thrombosis and endocarditis. The most common is SVD, defined as permanent intrinsic changes of the valve (i.e., leaflet tear, calcification, pannus deposition, flail or fibrotic leaflet) leading to degeneration and/ or dysfunction, which in turn may result in stenosis or intra-prosthetic regurgitation [1]. We describe the case of an 80-year-old woman with a medical history notable for severe aortic stenosis, severe mitral regurgitation and severe tricuspid regurgitation underwent surgical replacement with a concomitant aortic (Saint Jude Medical Epic 21mm) and mitral (Saint Jude Medical Epic 29mm) bioprosthesis implantation associated with tricuspid annuloplasty (with DeVega technique).
After about three years of good health, the patient presented to the emergency department with a chief complaint of worsening dyspnea for slight exercise. Transthoracic echocardiography revealed severe mitral regurgitation (EROA: 0.6cm2, regurgitant volume: 95mL) while transesophageal echocardiography revealed SVD with flail and perforation of bioprosthetic mitral valve posterior leaflet leading to severe mitral regurgitation. The patient was considered at high surgical risk so, after discussion in Heart Team, she was admitted in hospital and scheduled for a heterotopic balloon expandable aortic valve bioprosthesis (Edwards Sapien 3) transcatheter trans-septal implantation in mitral position.
Technical Resolution
The patient was brought in Cath lab where she underwent general anesthesia and orotracheal intubation. Through left common femoral vein access, pacing lead was placed in right ventricle for temporal pacing. Right common femoral vein access was achieved with femoral sheath 6 Fr and then an over-wire exchange with Swartz transseptal sheath 6 Fr was performed. Transesophageal echo-guided transseptal puncture with Brockenbrough needle 71cm was performed followed by septoplasty with Admiral Xtreme balloon. Then, during rapid pacing at 160bpm, an Edwards Sapien 3 29mm balloon-expandable bioprosthesis was implanted in mitral position. Hemostasis was achieved with Proglide device. No procedural complications were reported, and an excellent final echocardiographic and fluoroscopic result was achieved with trivial paravalvular leak.
The day after the procedure, transthoracic echocardiography revealed normal function of bioprosthetic valve in mitral position with mild paravalvular leak and normal tran prosthetic gradient (2mmHg); furthermore, it was revealed a trivial left-to-right shunt without any hemodynamic effect. The patient has been discharged from hospital three days after the procedure, receiving Warfarin therapy for three months, no complications were reported. At thirty-day ambulatory follow-up, the patient was in good health with significant improvement in quality of life. Transthoracic echocardiography, associated with structural 3D evaluation, revealed normal function of bioprosthetic valve in mitral position with normal trans-prosthetic gradient, the residual left-to-right shunt remains unchanged. Current pharmacological therapy was confirmed, and a three-months ambulatory follow-up was scheduled.
Clinical Implications
According to current European Society of Cardiology guidelines for the management of valvular heart disease, in bioprosthetic failure re-operation is recommended in symptomatic patients with a significant increase in trans-prosthetic gradient (after exclusion of valve thrombosis) or severe regurgitation (Class of Recommendation I, Level of Evidence C). Furthermore, transcatheter valve-in-valve implantation in the mitral and tricuspid position may be considered in selected patients at high risk for surgical re-intervention (Class of Recommendation IIb, Level of Evidence B) [2]. Edwards Sapien 3 valve is currently the only valve approved for valve-in-valve in mitral position, allowing patients at high or greater surgical risk to avoid an additional open-heart procedure. The TVT (Transcatheter Valve Therapy) registry published reports provides, to date, the wider data about transcatheter Mitral Valve-In-Valve (MViV) using Edwards Sapien 3 transcatheter valve for high-surgical risk patients with mitral bioprosthetic failure (Figure 1).
Figure 1:
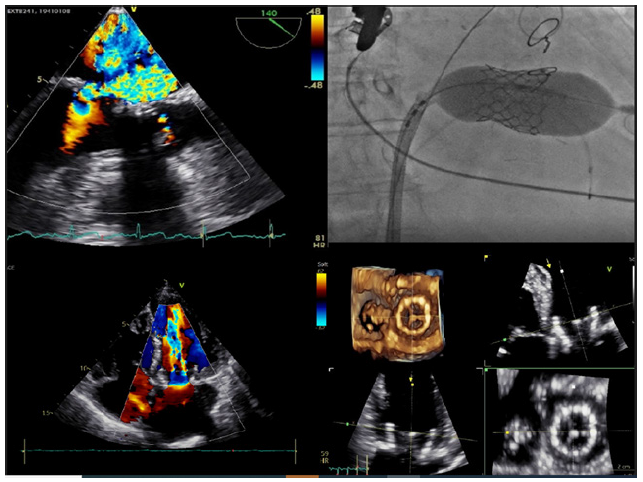
transapical (203, 13.3%) MViV implantation at 295 hospitals between 2015 and 2019. Procedural technical success (primary safety endpoint) was achieved for 1480 of 1529 patients (96.8%). All-cause mortality was 16.7% at 1 year (primary efficacy endpoint). In conclusion transcatheter MViV using Edwards Sapien 3 transcatheter heart valve is associated with high technical success, low 30-day and 1-year mortality, significant improvement of heart failure symptoms, and sustained valve performance [3]. Furthermore, the TMVR (Transcatheter Mitral Valve Replacement) registry sought to evaluate the outcomes of TMVR for patients with degenerated bioprostheses (valve-in-valve, ViV), failed annuloplasty rings (valve-in-ring, ViR), and severe mitral annular calcification (valve-in-mitral annular calcification, ViMAC). A total of 521 patients underwent TMVR (322 patients with ViV, 141 with ViR, and 58 with ViMAC). Trans-septal access and the Sapien valves were used in 39.5% and 90.0%, respectively. Overall technical success was excellent at 87.1% and it was higher after ViV compared with ViR and ViMAC. All-cause mortality with ViV at 30 days was 6.2%, significantly lower than surgical re-intervention (9.2-12.6%) [4].
Perspectives
In conclusion, mitral valve-in-valve heterotopic Edwards Sapien 3 transcatheter implantation provides excellent outcomes for patients with degenerated bioprosthesis despite high surgical risk. This procedure represents an excellent alternative to reintervention, most likely destined to become the gold-standard therapy for these patients.
References
- Capodanno D, Petronio AS, Prendergast B, Eltchaninoff H, Vahanian A, et al. (2017) Standardized definitions of structural deterioration and valve failure in assessing long-term durability of transcatheter and surgical aortic bioprosthetic valves: A consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg 52(3): 408-417.
- Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, et al. (2022) 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 43(7): 561-632.
- Whisenant B, Kapadia SR, Eleid MF, Kodali SK, McCabe JM, et al. (2020) One-year outcomes of mitral valve-in-valve using the SAPIEN 3 transcatheter heart valve. JAMA Cardiol 5(11): 1245-1252.
- Yoon SH, Whisenant BK, Bleiziffer S, Delgado V, Dhoble A, et al. (2019) Outcomes of transcatheter mitral valve replacement for degenerated bioprostheses, failed annuloplasty rings, and mitral annular calcification. Eur Heart J 40(5): 441-451.
© 2023 Castiello DS. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






