- Submissions

Full Text
Open Access Research in Anatomy
Structural and Cellular Characterization of Rectal Development in Goats: A Morphological, Histological, and Histochemical Study
Abhinov Verma1*, Farooqui MM2, Ajay P2 and Archana P2
1Anatomy Section, Indian Veterinary Research Institute, India
2Department of Veterinary Anatomy, UP Pt Deen Dayal Upadhyaya Pashu Chikitsa Vigyan Vishwavidyalaya, India
*Corresponding author: Abhinov Verma, Anatomy Section, Indian Veterinary Research Institute, Bareilly, India
Submission: July 18, 2025Published: August 01, 2025

ISSN: 2577-1922
Volume3 Issue1
Abstract
The rectal malformations are the most frequent and severe birth defects present in newborn animals. Many aspects of adult intestinal disease have an underlying developmental basis. The study was conducted to observe the sequential developmental changes of the rectum from its primordial stage till parturition. For this purpose, thirty-six (n=36) animals were used. The gross, histological, and histochemical examinations were performed on the rectum of the goat. The first morphological evidence of the rectum was notified on the 45th day of gestation. The rectal epithelium was transformed from stratified to simple columnar at the 75th day of gestation. The presence of rectal pits might be used for the identification of different segments of the large intestine in intrauterine life as well as to correlate it with pellet morphology in goats. The study uncovered a unique feature, the presence of villi in the rectum at 58th and complete degeneration up to150th days of fetal development. Among the connective tissue fibers, the reticular fibers appeared earliest followed by collagen and elastic fibers. The rectum showed an intense reaction for alkaline phosphatase, PAS, and AMPs.
Keywords:Histogenesis; Intestine; Ontogeny; Prenatal; Rectum
Introduction
Goats are the principal domesticated small ruminants in terms of total numbers and production of food and fiber products [1]. The development of intestine is important for the adult gastroenterologist to appreciate, because of the potential for these early life events to affect the responsiveness of the intestine to physiological or pathological challenges in later life [2]. Disruption in the development of the hindgut either environmentally or genetically can cause many disorders of the rectum and anal canal [3,4]. Moreover, many aspects of adult intestinal disease have an underlying developmental basis. The rectum plays a significant role in fecal continence and storage [5]. Prenatal development of the rectum has been studied in Sheep [6], Pig [7], Buffalo [8] and Camel [9], however very little attention has been paid towards systematic approach in goat rectum [10-12]. Besides this, the information collected in the present study will also fill the gap in the scientific literature on rectum development in goats.
Materials and Methods
The research work was carried out in the Department of Veterinary Anatomy, College of Veterinary Science, Mathura, U.P. (India). For this investigation, thirty-six (n=36) goat embryos or feti of either sex were collected from the local slaughter house, the Veterinary Clinical Complex, and the University Livestock Farm, Mathura. The age of procured fetuses was estimated using the formula derived by Singh [13] in goats. The formula was 1/3W=0.009 (t-30), where W=body weight (g) of fetus and t-age (days) of the fetus. In addition to this, the gestational age estimation based on phenotypic characteristics given by Njaa [14] in goats was also used in group I embryos or feti. The procured animals were divided into 3 groups: group I (0-50 days), group II (51-100 days), and group III (101-till term) with 12 feti in each group. All procedures were carried out in accordance with the protocol approved by the Institutional Animal Ethics Committee (IAEC) of DUVASU, Mathura (India). The abdominal cavity was opened by giving ventro-abdominal incision and abdominal cavity was exposed for morphological observations (through stereozoom microscope in group-I). Tissue sample of rectum was collected for histological and histochemical procedures and fixed in 10% neutral buffered formalin and in cold acetone. The tissues were processed in routine paraffin embedding technique [15] and the 5μm thick sections of tissues were obtained. These tissue sections were stained with Haematoxylin and Eosin [15] for general histoarchitecture, Masson’s trichrome stain [15] for collagen fibers, Gordon and Sweet’s method for reticular fibers [16], and Verhoeff’s stain [15] for elastic fibers. Micrometrical measurements were recorded with the help of Leica DM 750 computerized image analyzer. Each observation was recorded at six different places, and their mean, standard error was estimated. These sections were also subjected to Polysaccharides-Periodic Acid Schiff’s stain/ PAS stain [15], Acid mucopolysaccharides (AMPS)-Muller’s colloidal (hydrous) ferric oxide [15]. The fresh sample was fixed in cold acetone at 4 oC for histochemistry for using the Bound Lipids- Acetone Sudan Black-B method [17] and the Alkaline phosphatase enzyme-Modified Gomori method after Fredriksson [17]. The data generated by biometrical and micrometrical observations was subjected to statistical analysis to see the correlation between the various parameters and for the test of significance [18].
Result
Morphological observations
The rectum was differentiated from intestinal tube in group-I. The various stages of intestinal differentiation, viz., rotation, herniation, coiling, re-entry, and placement was noticed from the 23rd day to the 45th day of gestation. In 41-day-old feti, the caecal buldge demarcated the small and large intestines, while the rectum was identified first time by its position in the pelvic cavity at the 45th day of gestation (Figure 1a). It began ventral to the body of the first sacral vertebra and ended at the caudal end of the third coccygeal vertebra. A slight curve in the terminal part of the colon also demarcated it from the rest of the intestine at the 66th day, which became distinct at the 70th day of gestation (Figure 1b & 1c). On the 138th day of gestation, a distinct peritoneal fold was observed; the mesorectum was reflected from the cranial part of the rectum to the dorsal pelvic wall, while the caudal part remained retroperitoneal. The caudal part of the rectum was greater in diameter than the cranial one, termed the ampulla recti. In group I, the mucosal surface of the entire rectum was smooth, whereas in group II, from the 92nd day of gestation, longitudinal folds were observed. On the 115th day of gestation, the transverse fold (rectal columns) was also observed. On the 145th day of gestation, irregular branching of transverse and longitudinal folds was observed, which anastomosed to each other and resulted in the formation of rectal pits of various sizes (Figure 1d). Statistical analysis revealed that there was a significant increase (p≤0.01) in length and diameter of the rectum from group I to group III (0.43±0.019x0.04±0.002cm to 4.51±0.60 x 0.839±0.149cm).
Figure 1:Photographs of 45 day (Figure 1a), 66 day (Figure 1b), 70 day (Figure 1c)145 day (Figure 1d) old goat foetus showing descending duodenum (Dd), ascending duodenum (Da), caudal flexure (F), jejunum (J), ileum (I), caecum (C), descending colon (Co), rectum (R), curve (Cu) between colon and rectum, gubernaculum testis (Gt), anus (A), kidney (K) and left and right testis (Tl and Tr), longitudinal fold (L),transverse fold (T) and rectal pits (P).

Histological and histochemical observations
The detailed microscopic examinations revealed that the rectum showed certain key developmental features from early to late gestation. These events include differentiation of various tunics along with villi (formation and degeneration), goblet cells, intestinal glands, Gut-Associated Lymphoid Tissue (GALT), and the Enteric Nervous System (ENS). The wall of the rectum was composed of lamina epithelialis, propria submucosa, tunica muscularis, and tunica serosa at the 45th day of gestation (Figures 2-6).
Figure 2:Photomicrograph of 45 day (2a,2b), 50 day (2c), 58 day (2d) old goat foetal rectum showing the lumen (L), epithelium (E), basement membrane (B), propria submucosa (Ps), tunica muscularis (Tm) and tunica serosa (Ts), fibroblast cells (Fb), mesenchymal cells (Mc), luminal border (Lb), previllous ridge (Pr), villi (V), goblet cell (G), core of villi (Cv), blood vessels (Bv), superficial zone (S), deep zone (D) of propria submucosa, inner circular (C) and outer longitudinal (L) smooth muscle layers, connective tissue (Ct) in between muscle layers.

Figure 3:Photomicrograph of 70 day (3a), 75 day (3b), 80 day (3c) and 104 day (3d) old goat foetal rectum showing villi epithelium (E), core of villi (Cv), intraepithelial lymphocyte (Ly), supranuclear vacuolation (S) and cytoplasmic projections (Cp), villi (V), degeneration of villi (Vd), branching of villi (Vr), intestinal gland (Ig), branching of intestinal gland (Ib), intraepithelial lymphocytes in glandular epithelium (Ly) and propria submucosa (Ps), arteriol (A), vein (V) and arterial venous anastomosis (Av).

Figure 4:Photomicrograph of 107 day (4a), 127 day (4b), 138 day (4c,4d) old goat foetal rectum showing, intestinal gland (Ig), propria submucosa (Ps), ganglionic cell (Ga), inner circular (C) and outer longitudinal (L) smooth muscle layers and myenteric plexus (Mp) and nerve in tunica serosa (N), lymph nodule (Ln) and lymphoglandular complex (LC) lamina muscularis (Lm), anorectal junction (Aj), simple columnar epithelium (Ec), stratified squamous epithelium (Es).

Figure 5:Photomicrograph of 150 day (5a,5d), 115 day (5b), 127 day (5c), old goat foetal rectum showing rectal patches (Rp), lamina muscularis (Lm), inner circular (C) and outer longitudinal (L) smooth muscle layer, reticular fibers (Fr) in core of villi (Cv), propria submucosa (Ps) tunica muscularis (Tm) and Tunica serosa (Ts), collagen fibers (Fc) around artery (A) and internal elastic lamina (Ie) with in artery, elastic fibers (Fe) in artery (A).
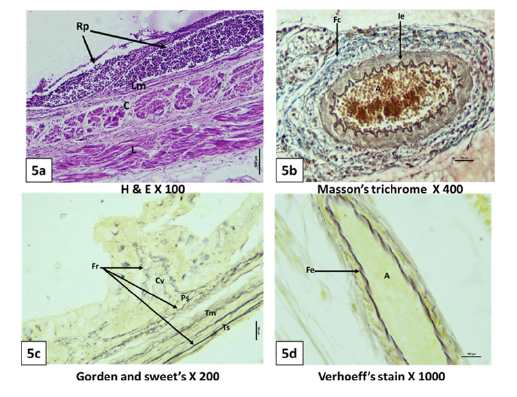
Figure 6:Photomicrograph of section of 97 day (6a, 6b),45 day (6c), 150 day (6d) old goat foetal rectum showing histochemical reactions for PAS, AMPs, bound lipids and alkaline phosphatase enzyme, respectively in luminal border (Lb), surface epithelium (E), goblet cell of villi epithelium (G) and intestinal glands (Ig), propria submucosa (Ps), tunica muscularis (Tm) and in tunica serosa (Ts).

Lamina epithelialis
The rectal epithelium was stratified columnar up to 48th day of gestation, predominately pseudostratified columnar on the 50th day, and became simple columnar from the 75th day of gestation onwards (Figures 2a-2c & 3b). The junction of the rectum and anus was clearly differentiated by the transformation of simple columnar epithelium into stratified squamous non-keratinized epithelium at the 115th day of gestation (Figure 4d). The luminal border of the cell showed a mild reaction for PAS, AMP, bound lipid, and alkaline phosphatase in group I as age advanced, these reactions became intense (Figure 6), (Table 1). One to two layers of fibroblasts were noticed just below the epithelium (Figure 2a & 2b). In group II, the epithelium was pseudostratified columnar with patches of simple columnar type at the 58th day of gestation (Figure 2d). The supranuclear and intranuclear vacuolations were noticed with a dominance of supranuclear vacuolations (Figure 3a). The luminal border was thick, highly eosinophilic, and showed filamentous cytolpasmic projections (Figure 3a). From the 75th day of gestation onward, simple columnar epithelium was noticed (Figure 3b). The basement membrane was distinct, continuous, and lightly eosinophilic in group I, which became highly eosinophilic at the 66th day, was interrupted at the 70th day, and became indistinct from the 72nd day of gestation onwards. The first evidence of gut-associated diffused lymphoid tissue (GALT) in the form of interepithelial lymphocytes was encountered on the 92nd day of gestation, which was more numerous in group III (Figure 4b). The average height of the epithelium in groups I, II, and III was 44.66±6.92, 40.77±1.76, and 26.11±2.23μm, respectively.
Villi
A novel observation revealed the morphogenesis and degeneration of villi in the rectum during prenatal life. The rectum showed epithelial invagination, with the previllous ridges at the 50th day of gestation, which got differentiated into villi at the 58th day of gestation (Figure 2c & 2d). The villi were cone, club, leaf, and treeshaped in group II, and leaf and cone-shaped in group III (Figure 2d & 3b). The core of the villi was comprised of densely packed mesenchymal cells, fibroblasts, microvasculature, and lymphocytes (Figure 2d). To support the core structures, the thin reticular fibers were observed on the 92nd day, which got converted into rete like on the 104th day of gestation (Figure 5c). The villi degeneration started at the 75th day of gestation as cone-shaped villi got transformed into club-shaped by the process of cell vacuolation and degeneration and almost disappeared at the 150th day of gestation (Figure 3b).
Goblet cells
The goblet cells were encountered on the 58th day of gestation in the form of cup- shaped cells, which had their nuclei towards the base, and the cytoplasm was lightly eosinophilic at this stage (Figure 2d). In Group-I and up to the 68th day in Group-II, goblet cells are located chiefly towards the base of villi while concentrated more towards the sides and tip of villi from the 70th day of gestation onwards. These cells showed a moderate to intense reaction for PAS and AMPs in groups II and III (Figure 6a & 6b), (Table 1).
Table 1:Histochemical reactions in rectum of prenatal goat at various stages of gestation.

-Negative, +Mild, 2+Moderate, 3+Intense, 4+highly Intense, *only in cell boundaries, I.G.= intestinal glands.
Intestinal glands/Crypts
The crypts were developed as an invagination of the surface epithelium into the underlying lamina propria submucosa at the 66th day of gestation (Figure 3b). Differentiating crypts were knob-like with pseudostratified types of epithelium. The goblet cells, intraepithelial lymphocytes, and undifferentiated cells were compartmentalized in the glandular epithelium (Figure 4b). The goblet cells exhibited moderate to intense PAS and AMP (Figure 6a & 6b). The undifferentiated cells showed several mitotic figures. The solid as well as canalized acini were noticed. A few canalized acini of the gland showed a branching pattern as well (Figure 3b). The gland was divided into lobes and lobules by connective tissue septae at the 75th day of gestation. In group III, the canalized acini had distinct simple columnar epithelium (Figure 3d). These acini were arranged linearly with the surface epithelium on the 115th day of gestation.
Lamina propria-submucosa
In group-I, the subepithelial connective tissue could be divided into two zones: the zone towards the surface epithelium, which contained densely packed cells in a radiating manner, was the superficial zone, and the zone towards the tunica muscularis, which had loosely packed cells, was the deep zone (Figure 2c). Both zones were composed of mesenchymal cells, fibroblasts, differentiating lymphocytes, blood vessels, and reticular fibers (Figure 2c). The reticular fibers were thin and isolated in group I and became thick and continuous in group III at the 115th day of gestation (Figure 5c). In group II, between 51 and 75 days of gestation, the propria submucosa had numerous fibroblasts in between the mesenchymal cells, and the occurrence of blood vessels also increased. In between the mesenchymal cells, scattered nucleated RBCs and differentiating lymphocytes were distinctly evident at the 58th day of gestation. The thin, isolated collagen fibers were noticed for the first time on the 97th day of gestation, which transformed into wavy bundles on the 127th day of gestation. Distinct lymphocytic infiltration was noticed for the first time close to the anorectal junction at 92 days of gestation. The sparsely arranged lymph nodules observed close to the intestinal glands in the propria-submucosa at the 127th day of gestation could be termed a lymphoglandular complex (Figure 4a). The aggregated lymph nodules or rectal patches were noticed at the 150th day of gestation (Figure 5a). The scattered ganglionic cells were also
encountered on the 107th day of gestation, which indicates the first evidence of the establishment of ENS (enteric nervous system) (Figure 3d). The Arterial Venous Anastomosis (AVA) was noticed in the propria-submucosa close to the anorectal junction on the 104th day of gestation (Figure 3c). The lamina propria and tunica submucosa were demarcated for the first time by a layer of lamina muscularis in group III at the 138th day of gestation (Figure 4c). The connective tissue cells of the propria submucosa showed a mild reaction to PAS and AMP in group I and a moderate in groups II and III (Table 1). The connective tissue cells showed a moderate to intense reaction for bound lipids and a faint reaction for alkaline phosphatase. The average thickness of the propriasubmucosa in groups I, II, and III was 71.51±4.78, 125.73±11.48, and 135.49±9.16μm, respectively.
Tunica muscularis
Few mesenchymal cells of the propria submucosa cells got elongated with highly eosinophilic cytoplasmic extensions, indicating differentiation of the muscular layer on the 45th day, and on the 50th day of gestation, a distinct circular muscle layer was evident for the first time (Figure 2a & 2c). The outer longitudinal muscle fibers were encountered at the 58th day of gestation (Figure 2c). The outer longitudinal muscle fibers were more compactly arranged than the inner circular muscle fibers and exhibited a bundle-like or fasiculi-like appearance (Figure 2d & 3b). Both the muscle layers showed intensely bound lipid and alkaline phosphatase reactions (Figure 6c & 6d). The loose connective tissue between both muscle layers contained mesenchymal cells, fibroblasts, and small blood vessels. Differentiating myenteric plexus was noticed at the 82nd day, which got distinct on the 107th day of gestation and was comprised of ganglionic and supporting cells (Figure 3d). The smooth muscle cells showed mild reactions to PAS and no reaction to acid mucopolysaccharides. The outer longitudinal smooth muscle layer exhibited a more positive reaction than the inner circular layer for bound lipids. The average thickness of the tunica muscularis in groups I, II, and III was 16.44±0.27, 89.73±12.96, and 100.52±6.26μm, respectively.
Tunica serosa
In group I, the tunica serosa was comprised of a layer of simple squamous epithelium, the mesothelium, and loosely arranged connective tissue beneath the mesothelium (Figure 2a). The mesothelium was interrupted on the 45th day and became continuous from the 50th day of gestation onwards (Figure 2c). The arterial venous-anastomosis was noticed close to the anorectal junction at the 50th day, which got distinct at the 104th day of gestation (Figure 3c). The blood vessel supplying the nerve trunk, the vasa-nervi, was evident on the 107th day of gestation (Figure 3d). The tunica media of large arteries and veins contained thick bundles of collagen fibers, while the elastic lamina was formed by thick elastic fibers (Figure 5b & 5d). Differentiating nerve trunk was observed at the 58th day of gestation, which got clearly distinct from the 75th day of gestation onwards. The mesothelium underwent weak PAS and AMP reactions. The RBCs in the blood vessels of serosa were moderate to intensely positive for bound lipid reactions. The average thickness of the tunica serosa in groups I, II, and III was 15.92±0.25, 76.14±12.58, and 96.29±4.54μm, respectively. Micrometrical analysis showed a continuous increase in the thickness of all strata of the wall of the rectum except the thickness of the epithelium, which decreased as the age of gestation advanced (Figure 7). The thickness of the propria-submucosa increased about 1.5 times from groups I to II and was minutely amplified from groups II to III. Propria- submucosa was thickest among all strata of the wall of the rectum (Figure 8). The thickness of tunica muscularis increased about 5.0 times from group I to group II. The thickness of Tunica serosa increased gradually from group I to group II, and it increased about 5.0 times from group II to group III.
Figure 7:Bar diagram showing changes in the thickness of different layers of rectum in personal goat group I, II and III.
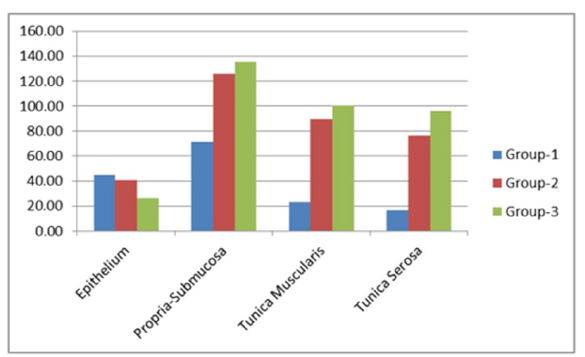
Figure 8:Bar diagram showing percentage proportion in the thickness of different layers in the wall of rectum in group III.

Discussion
It was vital to fully explain key developmental events that may be crucial for the establishment of anatomo-embryologicfetal standards for goats. The identification of the rectum through its location in the pelvic cavity and the presence of a curve close to the descending colon showed full agreement with Getty [19] in ruminants and Nakashima [5] in humans, while there was variation with Siyag [20], who could not observe any clear demarcation between the colon and rectum in goats. The function of the ampulla recti might be to regulate the movement of fecal material into the anus for expulsion, as stated by Nakashima [5] in humans. The presence of mesorectum near term tallied with the findings of Prakash [21] in goat kids. The presence of longitudinal and transverse folds in the mucosal surface is attributed to fecal palate behavior, as demonstrated by Badawi [22] in goats and donkeys and Bello [23] in camel foeti. Significant increases in all biometrical parameters of the rectum from groups I to III were in full agreement with Raghav [12] in goat and Singh [24] in buffalo foeti [25-27] in duodenum, caecum, and jejunum of goat foeti, respectively. In our findings, the transformation of stratified epithelium to simple columnar was completed at the 82nd day of gestation; however, other researchers identified this event at the 174th day of gestation, in the second trimester, and at the 120th day of gestation in buffalo, camel, and goat, respectively [12,23,24]. The literature related to cytoplasmic streemers in embryonic life was very few, and the information available in postnatal periods [28] correlates it with the secretary function of the rectal wall. The presence of predominant supranuclear vacuolations in epithelial cells was compared to the reports of Raghav [12] in goat feti and might be the reserve cells replenishing other cell types. GALT is the main component of MALT. It is a complex infrastructure of immune cells [12]. The presence of GALT components in prenatal life might be correlated with markers for the development of innate immunity. Lymphocytes are potent, rapidly activated cytolytic and immunoregulatory effectors that can protect host tissues from infection, cell transformation, and uncontrolled infiltration by systemic cells [29]. The appearance of goblet cells at the 58th day in the rectum, in the duodenum at the 55th day, in the in the jejunum, ileum, and colon (66th day), and in the caecum at the 75th day of gestation [27,30] indicated the caudocranial development of the intestine. As the age of the foeti increased, the occurrence of goblet cells was also enhanced [24,31,32] and concentration was more towards the base of villi, as described by Raghav [12] in goat foeti. The goblet cell secretions, such as mucin, were the first physical barrier for gut immunity and are responsible for the production and maintenance of the protective mucus blanket [33]. The rectal villi demonstration on the 58th day was in close proximity to the findings of Raghav [12] on goat foeti at the 50th day, but far away in cattle and buffalo foeti at 3 months and the 119th day of gestation, respectively [24,34]. Variation in the appearance of villi might be due to differences in species, breed, nutritional status, and environment. The presence of a branching anastomosing pattern in the mucosa might be a novel result for identifying different segments of the large intestine in intrauterine life. Degeneration of villi started on the 97th day and ended on the 150th day. However, previous researchers [12,24] noticed these events in goat and buffalo foeti on the 64, 161, and 150 and 272 days of gestation, respectively. The first evidence of intestinal glands (on the 66th day) was nearest to the findings of Raghav [12] at the 69th day of gestation in goat feti. The Lymphoglandular Complex (LGC) was structurally similar to the observations of Kapoor [35] in buffalo feti. The lamina muscularis appeared at the 138th day of gestation in buffalo foeti [31] at the third trimester in one humped camel [23] and at the 131st day of gestation in goats [12]. Our findings complement the previous report of Raghav [12] who also noticed better-developed lamina muscularis at the caudal portion of the rectum, indicating caudocranial development of the large intestine. The appearance of connective tissue fibers (reticular, collagen, and elastic fibers) was almost similar to the previously published study on goats [12]. The large blood vessels and neuronal elements observed in the propria submucosa were similar to those in buffalo feti at the 198th day of gestation [24]. Meisners plexus was observed on the on the 107th day, the 198th day in buffalo [36] and the 91st day in humans [31]. Aggregated lymph nodules were encountered on the 127th day of the present study as the 120th day in goat foeti [37], the 70th day in sheep [38] the 195th day of gestation in buffalo foeti [16], and the 3rd trimester in one humped camel [5]. Rectal patches represent the active immunological defense of the intestinal mucosa [37,39,40].
The presence of arterial venous anastomosis in the propria submucosa correlated with an important role in the regulation of blood pressure and blood flow into the capillary bed [41]. In Tunica muscularis, the inner circular and outer longitudinal layers appeared on the 58th day, while Raghav [12] in goat foeti and Singh [24] in buffalo foeti noticed these layers on the 78th and 119th days of gestation, respectively. The presence of loose connective tissue between muscle layers agreed with the findings of Raghav [12]in goat foeti in the mid-gestation period. The development of ENS was first evident at 82 days in the form of a myenteric plexus, as noticed by Raghav [12] in goat foeti. The composition of Tunica serosa in our study tallied the findings of Raghav [12] in goat foeti, Kumar [31] in buffalo foeti, and Eurrell and Frappier [41] in adult domestic animals. The micrometrical analysis of the thickness of all strata of the rectum tallied with the findings Ramakrishna and Tiwari [42], Raghav [12] in goat foeti, and Singh [24] in buffalo foeti. In the present study, the PAS and AMPs reactions in the epithelial and goblet cells were compared to the findings of Raghav [12], Verma, et al. [43] in goat foeti, and Kumar [31] in buffalo foeti. Connective tissue cells of the propria submucosa and tunica submucosa showed mild PAS and AMPs reactions, as noticed by Raghav [12] in goat foeti [44].
Similarity with our study
Deplancke and Gaskins stated that acidic mucins were predominant in the large intestine. The reaction for bound lipids was similar to the findings of Raghav [12] in goats, while there was partial agreement with Kumar [31] in buffalo feti. The results for the alkaline phosphatase reaction in the present study had similarities to the findings of Raghav [12] in goats. Intestinal alkaline phosphatase has an important role in gut mucosal defense. All the histochemical reactions were intense in group III, which might be an indication of the functional status of the rectum just prior to birth.
Conclusion
The presence of rectal pits and the branching anastomosing pattern of the mucosa were novel findings of this research for identifying different segments of the large intestine during intrauterine life as well as correlating them with pellet morphology in goats. The rectum was in a functional stage just prior to birth, as it showed intense reactions for alkaline phosphatase, PAS, and AMPs and the presence of GALT, LGC, and ENS.
Acknowledgement
Sincere thanks to Department of Pathology, College of Veterinary Science and Animal Husbandry, DUVASU, Mathura for support in photography of histological sections for this study.
References
- Bello A, Suleiman HM, Bodinga HA, Theresa EC (2020) Age related changes in the development of large intestine in red sokoto goats. Open Access Research in Anatomy 2(2): 1-10.
- Drozdowski LA, Clandinin T, Thomson AB (2010) Ontogeny, growth and development of the small intestine: Understanding pediatric gastroenterology. World Journal of Gastroenterology 16(7): 787-799.
- Lee JM, Kim NK (2018) Essential anatomy of the anorectum for colorectal surgeons focused on the gross anatomy and histologic findings. Annals of Coloproctology 34: 59-71.
- Wang YH, Wiseman J (2022) Anatomy, abdomen and pelvis, rectum.
- Nakashima J, Zulfiqar H (2022) Embryology, rectum and anal canal. Stat Pearls, USA.
- Karadag H, Ozmen E, Vilmaz S, Dine G, Teke (1994) Morphogenesis of small intestine of ovine fetus. FV Saglik Bil Dergisi 8: 7-12.
- Sangild PT, Fowden AL, Trahair JF (2000) How does the foetal gastrointestinal tract develop in preparation for enteral nutrition after birth? Livestock Production Science 66: 141- 150.
- Singh O, Roy KS, Sethi RS, Kumar A (2009) Histomorphological study on duodenum of buffalo during prenatal development. Indian Journal of Animal Sciences 79(6): 571-573.
- Bello A, Onyeanusu BI, Sonfada ML, Adeyanju JB, Umaru MA (2012) A biometric study of the digestive tract of one-humped camel (Camelus dromedarius) fetuses. Scientific Journal of Zoology 1: 11-16.
- Chima NI, Nwagbo ED (2009) Changes in morphological features of duodenum and jejunum of prenatal and postnatal West African dwarf goats (Capra hircus). Tropical Veterinarian 27: 1-10.
- Gautam AK, Mishra UK (2016) Development of intestinal immunity in prenatal stages of black bengal goat. Journal of Cell and Tissue Research 16(1): 5527-5530.
- Raghav S (2016) Histomorphological and histochemical studies on prenatal development of goat large intestine.
- Singh Y, Sharma DN, Dhingra LD (1979) Morphogenesis of the testis in goat. Indian Journal of Animal Science 49(11): 925-931.
- Njaa BL (2012) Kirkbride’s diagnosis of abortion and neonatal loss in animals. John Wiley and Sons, USA.
- Luna LG (1968) Manual of histological staining methods of the armed forces institute of pathology. (3rd edn), McGraw-Hill, USA.
- Suvarna SK, Layton C, Bancroft JD (2019) Bancroft’s theory and practice of histological techniques. Elsevier Limited, UK.
- Pearse AGE (1968) Histochemistry: Theoretical and practical. Churchill Livingstone, UK.
- Snedecor GW, Cochran WG (1994) Statistical methods. Oxford and IBH Publishing Company, India.
- Getty R (2012) Sisson and grossman’s the anatomy of the domestic animals. India.
- Siyag RK, Dangi A, Thanvi PK, Pura R, Yogi VK, et al. (2022) Gross anatomical studies on the rectum of goat (Capra hircus). The Pharma Innovation Journal 11(7): 283-286.
- Prakash A (1998) Gross, histoarchitectural and histochemical studies on the intestine of goat (Capra hircus).
- Badawi H, Yousria A, Elrahman ABD, Salem AO, Mohamed AM (1999) Morphological studies on the colon and rectum of some domestic animals with special reference to the end form of the fecal matter. Association of Veterinary Medical Journal 40(80): 32-55.
- Bello A, Onyeanusu BI, Sonfada ML (2015) Embryonic Differentiation of the rectum of one humped camel (Camelus dromedarius): A histomorphology. Direct Research Journal of Agriculture and Food Science 3(6): 127-131.
- Singh O, Roy KS, Sethi RS, Kumar A (2012) Development of large intestine of buffalo. Indian Journal of Animal Sciences 82(10): 83-100.
- Verma A, Farooqui MM, Prakash A, Pathak A, Singh SP, et al. (2020) Topographical and biometrical anatomy of duodenum in prenatal goats. Indian Journal of Small Ruminants 26(2): 214-218.
- Verma A, Farooqui MM, Prakash A, Pathak A, Singh SP, et al. (2021a) Age related variation in gross anatomy of caecum in prenatal goat (Capra hircus). Indian Journal of Veterinary Anatomy 33(2): 156-157.
- Verma A, Farooqui MM, Prakash A, Pathak A, Singh SP, et al. (2021b) Gross anatomical observations on the jejunumin prenatal goat (Capra hircus). Indian Journal of Veterinary Anatomy 33(1): 4-6.
- Fisinin VI, Surai P (2013) Gut immunity in birds: Facts and reflections. Agricultural Biology 4: 3-25.
- Montalban A, Chaparro M, Gisbert JP, Bernardo D (2018) The innate immune system in the gastrointestinal tract: Role of intraepithelial lymphocytes and lamina propria innate lymphoid cells in intestinal inflammation. Inflammatory Bowel Diseases 24(8): 1649- 1659.
- Verma A, Farooqui MM, Prakash A, Pathak A, Singh SP, et al. (2021c) Histogenesis of jejunum in prenatal goat. Haryana Veterinarian 60(1): 47-50.
- Kumar R (2006) Anatomy and histomorphological studies on large intestine during prenatal development in buffalo.
- Devi HR, Pfoze K, Singh TN, Singh YI (2018) Morphogenesis of colon in human foetuses. IOSR Journal of Dental and Medical Sciences 17(9): 62-66.
- Kim JJ, Khan WI (2013) Goblet cells and mucins: Role in innate defense in enteric infections. Pathogen 2(1): 55-70.
- Asari M, Kashiwazaki N, Kawaguchi N, Fukaya K, Kano Y (1986) Developmental changes in the inner surface structure of the bovine large intestine. Acta Anatomica (Basel) 127(2):137-141.
- Kapoor K, Singh O (2015) Gross morphological studies on the gut associated (galt) lymphoid tissue of buffalo foetuses. Indian Journal of Veterinary and Animal Sciences Research 44(6): 427-433.
- Singh S, Shariff A, Roy TS, Kumar H (2013) Prenatal development of the myenteric plexus in human sigmoid colon. Journal of Morphological Sciences 30(3): 156-166.
- Indu VR, Lucy KM, Ashok N, Maya S (2018) Prenatal development of rectal patch in large intestine of goats. Indian Journal of Animal Research 52(2): 232-234.
- Aleksandersen M, Hein WR, Landsverk T, Mcclure S (1990) Distribution of lymphocyte subsets in the large intestinal lymphoid follicles of lambs. Immunology 70(3): 391-397.
- Liebler EM, Pohlenz F, Cheville N (1988) Gut-associated lymphoid tissue in the large intestine of calves. Veterinary Pathology 25(6): 509-515.
- Alboghobeish N (2005) Morphological and histometrical studies of lymphatic patches of buffalo large intestine in different ages. Iranian Journal of Veterinary Research 6(3): 35-41.
- Eurell J, Frappier B (2006) Dellmann’s textbook of veterinary histology. Blackwell Publishing, USA.
- Ramkrishna V, Tiwari GP (1979) Prenatal intestinal histology and histochemistry in the goat. Acta Anatomica (Basel) 105(2): 151-156.
- Verma A, Farooqui MM, Prakash A, Pathak A, Singh SP, et al. (2022) Histochemical characterization of duodenum at early, mid and late prenatal period in goats. Indian Journal of Experimental Biology 60(12): 939-945.
- Verma A, Farooqui MM, Prakash A, Pathak A, Singh S (2023) Embryonic differentiation of intestine in Indian goats (Capra hircus). Anatomia, Histologia, Embryologia 52(4): 627-635.
© 2025 Abhinov Verma. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






