- Submissions

Full Text
Open Access Research in Anatomy
Direct Puncture Endoscopic Assisted Gastrostomy: An Alternative Technique, Case Report
Manuel Cadena1, Jaime Solano3, Andrés Salazar2, José Antonio Hakim1, Maria Jose Vergara3 and Arturo Vergara4
1Departamento de Cirugía general, Hospital Universitario Fundación Santa Fe de Bogotá, Colombia
2Departamento de Gastroenterología, Hospital Universitario Fundación Santa Fe de Bogotá, Colombia
3Medico General Pontificia UniversidadJaveriana, Colombia
4Servicio de Soporte Metabólicoy Nutricional, Hospital Universitario Fundación Santa Fe de Bogotá, Colombia
*Corresponding author: Manuel Cadena, Departamento de Cirugía general, Hospital Universitario Fundación Santa Fe de Bogotá, Colombia
Submission: December 11, 2017; Published: January 10, 2018

ISSN: 2577-1922 Volume1 Issue3
Introduction
Enteral feeding benefits are worldly renown, severely ill patients and oncological patients have better outcomes, survival rates and improvement in-quality life standards [1-6]. Gastrostomy is the procedure ideal for management of these chronic and ill patients [7]. Within many available techniques PEG tube is an effective access to provide enteral feeding for patients with impaired oral intake, and now a day's oncologic patients have become a demand group for enteral nutrition [2,5,7-10], especially the ones coursing with head, neck and esophagealneoplasia. 33-69% of patients that need chemo-radiotherapy for upper aero digestive tract require PEG tube placement [11-13]. This group disserves particular attention since tumoral seeding in gastrostomy stomas have been reported [13-17], primary from the oropharynx, hypopharynx, oral cavity and larynx. Being the pull technique the most associated with metastases reports, reason why new methods for gastrostomy placement have been studied, especially for the ones that require pre-surgical feeding or for nutritional feeding after mayor head and neck surgical intervention [12,18,19]. With this purpose, general surgery and gastroenterology departments of the Fundación Santa Fé University Hospital of Bogotá Colombia presents an alternative technique: Direct puncture endoscopic assisted Gastrostomy reducing the risk of tumoral cell implant at the path and stoma.
Materials and Methods
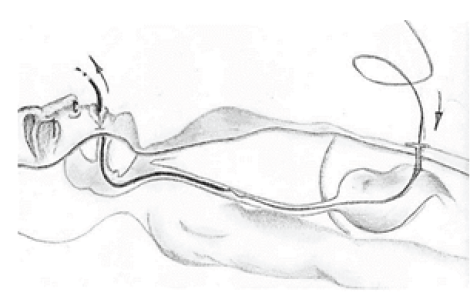
Traditional technique for percutaneous gastrostomy PEG was described in 1980 by Gauderer et al. [7,20]. Various methods of PEG tube placement have been reported Gauderer-Ponsky, Sacks- Vine, Russell and radiologic-assisted techniques [21,22]. The “pull” placement technique is the most commonly practiced worldwide [7,23,24]. In this technique, the tube is moved through the endoscopy to be recovered by simple traction trough the abdominal wall (Pull technique) [21,23,25] (Figure 1 & 2). This Technique is widely standardized and used but has a disadvantage for head and neck and esophageal oncologic patients, since goes through the primary lesion as an obligated pathway for the tube placement. Therefore, carries tumoral cells trough the path of the tube and secondary metastasis could be present [8,13-17].
Figure 1: Punture and capture of the metallic guidance at the abdominal Wall.guided through trans illumination by the endoscope.
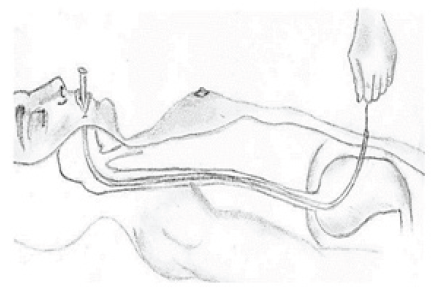
Figure 2: With the metallic guidance de tube is driven through the esophagus until abdominal Wall is reached.
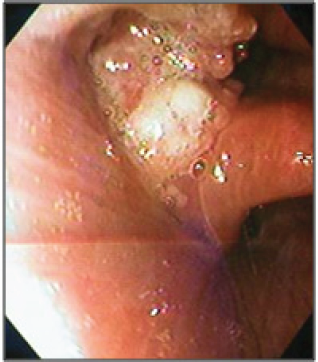
With this purpose, we present the direct puncture endoscopic assisted Gastrostomy In this technique, the surgeon, with the endoscopic help, uses an inverse technique, avoiding the pass of the tube trough the head and neck tumor, reducing the risk of tumoral cell implant at the path and stoma. The technique has two principal steps: first set the gastric wall adjacent to the abdominal wall, second passing the tube trough the gastric lumen (Figure 3 & 4). To illustrate this technique, we present the case of patient coursing with and squamous cell carcinoma of the piriform sinus (Figure 5), who was going to be taken to preoperative radiotherapy, with the spectated repercussion in food intake intolerance and secondary dysphagia, that required the placement of a gastrostomy tube prior to these interventions.
Figure 3: Setting gastric wall adjacent to the abdominal wall. Guided with endoscope.
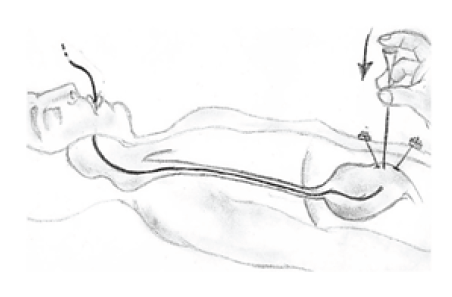
Figure 4: Setting the tube to its correct position.
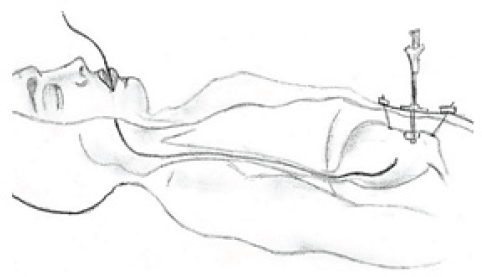
Figure 5: Endoscopic view of the scquamous cell carcinoma of the piriform sinus.
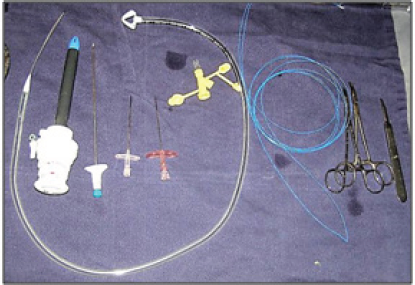
Materials
1. Gastrostomy tube: pull gastrostomy kit. That can be changed by a changeable
2. High French diameter needles
3. laparoscopic trocar no.12mm. disposable endoscope
4. Basic surgery kit: scalpel, Kelly, material, and tissue scissors.
5. Propylene suture 0.
Procedure
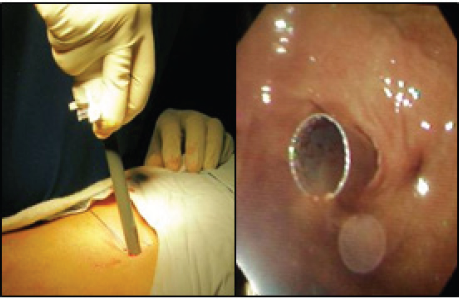
Figure 6: Trans illumination of the puncture site.
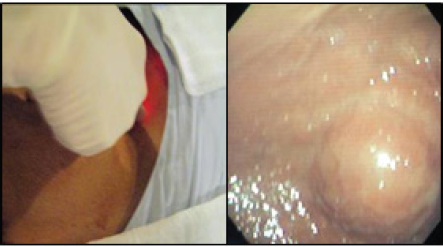
Figure 7: Materials.
Under general anesthesia, previous procedure asepsis and antisepsis was performed. Initial upper endoscopy revealed by trans illumination the puncture site, and local anesthesia with lidocaine was applied. As anatomical reference an imaginary triangle was used within the xiphoid process, belly button and the left costal margin with anterior axillar line (Figure 6 & 7). Second step consist in placing the gastric wall adjacent to abdominal wall. with endoscopic aid, the abdominal wall was puncture with 2 thick needles until reaching the gastric lumen, the propylene suture was inserted through one of the needles and with endoscopic wire loop recovered so an "U" stitched was used, passing the suture trough the other needle. The suture was stitched in the abdominal wall skin. (The wire loop function could be replaced by an endo catch) (Figure 8) The Abdominal and gastric wall suture can be with 2 or 4 stiches. In this case 2 stiches with 2cm of space within them was enough (Figure 9) taking into a count that 4 points to close together could affect vascular supply to the gastric wall; that could cause necrosis and a difficult stoma wound healing and as a long-term complication a gastro cutaneous fistula bigger than the tube diameter. In the third step the laparoscopic trocar was placed through the skin reaching the gastric lumen, so the tube could be advanced. An incision in the skin wasmade and the trocar was pushed through the abdominal wall and gastric wall with the endoscope guidance to ensure the position between the stiches placed before (Figure 10).
Figure 8: Abdominal and gastric wall suture with a U stitch.
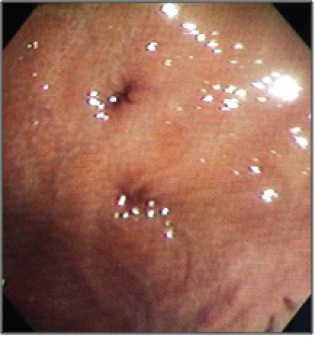
Figure 9: Gastric Wall with suture placement.
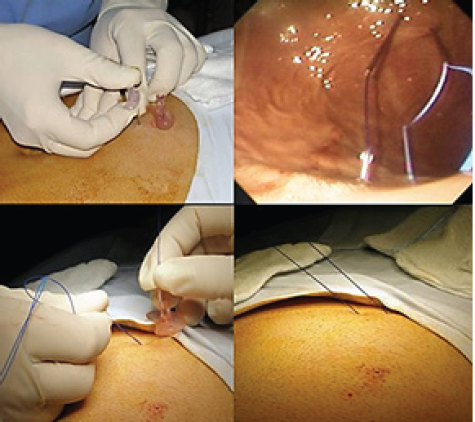
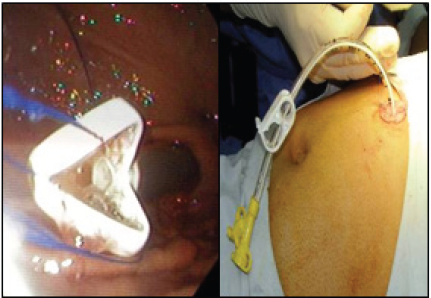
Figure 10: Laparoscopic trocar placement trough gastric lumen between the stiches.
Finally, gastrostomy tube is pushed through the trocar and fixed to the abdominal wall as usual. Procedure time was 30 minutes; time that surely can be shorten ones an adequate learning curve is achieved. Nutrition was started 24 hours after procedure. Both functional as esthetic expectations were the ones desired for this type of procedures and this type of patients (Figure 11). After 2weeks'patient went to ambulatory consult to stitches remove with no complications and with good feeding tolerance trough the gastrostomy.
Figure 11: Gastrostomy in right position.
Discussion
Oncologic patients require in some cases preoperative radiotherapy, although necessary it expose the patient to several risks as mucositis, dysphagia, parageusia, anorexia and dehydration [6,7,9,18,26]. Approximately 60% of patient's present weight loss and chronic malnutrition affects approximately 20-57% of patients with head and neck neoplasia, reason why and early enteral feeding must be started to avoid or at least reduce complications and comorbidities [1,11,12,18,19,26-29]. Nutritional assessment and intervention should be an integral part of treatment since this complication can lead to compromised treatment efficacy. Today the PEG technique has reduce the use of nasogastric tubes and open gastrostomy tube placement due to reductions in major complications, patient discomfort, days spent in the hospital, and costs [23,30]. Mortality is less than 1%, mayor complications as early tube extrusion, Buried bumper syndrome, PEG site metastasis, and visceral perforation represent 1% and minor complications as peristome infection, periostomal leakage, late tube extrusion, impacted lumen cellulitis, ileus, and hematoma represent 5% to 15% [31-35].
By 1989, Preyer and Thul reported the first case of upper aero digestive tract cancer metastatic to a PEG site [16], since then several isolated case reports were described until 2007 were all existing cases were reviewed, Pathologically proven periostomal metastases were located in 44 patients specifically in the abdominal wall in 28 patients (63%), in the gastric wall in 3(7%), and in both walls in 13 (30%) [17]. the latest literature review in 2013 showed stomal metastases after percutaneous endoscopic gastrostomy (PEG) in 42 patients being the oropharynx the most common site with (40%), hypopharynx (29%) oral cavity (17%), and larynx (14%), the method of PEG tube insertion was documented in 29 cases, with 28 (96.6%) reporting use of the ("pull") technique [13,36,37]. Recently Ellrichmann et al. [37] published a prospective study of 50 cases of oropharyngeal and esophageal malignancies that showed malignant tumor cells present on 22.5% from PEG tube or brush cytology of the incision site immediately after PEG placement with pull technique [36,38].
Taking this into a count, there is the need to find a cost-effective and useful alternative, that avoids passing through the path of tumor site different from conventional open gastrostomy or laparoscopic, that have an important repercussion in costs, prolonged anesthesia time, need of a laparotomy, and prolonged hospital stay. Surgery innovations come as solutions to problems in the clinical practice that need to be solved. Indeed, the professional must look for new alternatives or modify existing ones so that limitations and deficiencies of existing ones can be improved. This is the case of the puncture gastrostomy, procedure that pretends to avoid the complication of tumoral seeding's of head and neck tumors in the path of the tube.
Procedure that could be used not only in this patients but In case of missing endoscopy kit resources, but having the elements of the open technique without the need of a laparotomy [13,24,30,39]. This technique is easily reproducible, cost are the same or below endoscopic or laparoscopic technique and any surgeon with endoscope help can perform this technique without major complications just by following basic security and skill used in any surgery. For example, eco-endoscope assisted puncture could aid to avoid vascular lesion in the gastric wall. That why this procedure has several advantages compared to open and endoscopic-gastrostomy.
Although in this case we recommended general anesthesia, surgical time is like endoscopic and surely less than open. Also, general anesthesia has de advantage of sedation and protecting the airway, what makes endoscopic approach easier, nevertheless once one technique is well developed and more experience is acquired, locally assisted anesthesia could be used. These procedures require to be developed in the operating room, facility that permits if needed converting the procedure to an open fashion in the context of a complication. Making it easier taking into a count that there is no need to move the patient or equipment to operating room. This method, as the open gastrostomy places the gastric wall adjacent to the abdominal wall, avoiding leaks that usually are present in endoscopic pull gastrostomy. Although patient goes through general anesthesia, this procedure can be considered as an ambulatory surgery, patient can be discharged the same day after a short period of observation and nutritional intake trough gastrostomy 24 hours after the procedure.
Conclusion
Tumoral seeding in gastrostomy stoma is rare and literature has largely been limited to isolated case reports. The small number of cases reported, and lack of existing large patient series or prospective studies has excluded adequate examination. Puncture gastrostomy is presented as an alternative to traditional technique in cases where complications as tumoral seeding in the stomas must be avoided; also it might be used in other cases where open gastrostomy and endoscopic advantages can be fusion. 4% of patients diagnosed with PEG site disease either had simultaneous or subsequent loco regional or distant metastatic disease, suggesting that PEG site metastases may be a marker of aggressive tumor behavior. Since its implementation doesn't require big technical efforts apart from the basic supplies present in almost all institutions, it's a technique easily reproducible.
References
- Marik PE, Zaloga GP (2001) Early enteral nutrition in acutely ill patients: a systematic review. Crit Care Med 29(12): 2264-2270.
- Andersen HK, Lewis SJ, Thomas S (2006) Early enteral nutrition within 24h of colorectal surgery versus later commencement of feeding for postoperative complications. Cochrane Database Syst Rev 18(4): CD004080.
- Minard G, Kudsk KA (1994) Is early feeding beneficial? How early is early? New Horiz 2(2): 156-163.
- Wereszczynska-Siemiatkowska U, Swidnicka-Siergiejko A, Siemiatkowski A, Dabrowski A (2013) Early enteral nutrition is superior to delayed enteral nutrition for the prevention of infected necrosis and mortality in acute pancreatitis. Pancreas 42(4): 640-646.
- Woo SH, Finch CK, Broyles JE, Wan J, Boswell R, et al. (2010) Early vs delayed enteral nutrition in critically ill medical patients. Nutr Clin Pr 25(2): 205-211.
- Bechtold ML, Matteson ML, Choudhary A, Puli SR, Jiang PP, et al. (2008) Early versus delayed feeding after placement of a percutaneous endoscopic gastrostomy: A meta-analysis. Am J Gastroenterol 103(11): 2919-2924.
- Colasanto JM, Prasad P, Nash MA, Decker RH, Wilson LD (2005) Nutritional support of patients undergoing radiation therapy for head and neck cancer. Oncology 19(3): 371-379.
- Cruz I, Mamel JJ, Brady PG, Cass-Garcia M (2005) Incidence of abdominal wall metastasis complicating PEG tube placement in untreated head and neck cancer. Gastrointest Endosc 62(5): 708-711.
- Cady J (2007) Nutritional support during radiotherapy for head and neck cancer: the role of prophylactic feeding tube placement. Clin J Oncol Nurs 11(6): 875-880.
- Sinclair JJ, Scolapio JS, Stark ME, Hinder RA (2001) Metastasis of head and neck carcinoma to the site of percutaneous endoscopic gastrostomy: case report and literature review. JPEN J Parenter Enteral Nutr 25(5): 282-285.
- Jham BC, Reis PM, Miranda EL, Lopes RC, Carvalho AL (2008) Oral health status of 207 head and neck cancer patients before, during and after radiotherapy. Clin Oral Investig 12(1): 19-24.
- Ravasco P, Monteiro-Grillo I, Marques Vidal P, Camilo ME (2005) Impact of nutrition on outcome: A prospective randomized controlled trial in patients with head and neck cancer undergoing radiotherapy. Head Neck 27(8): 659-668.
- Huang AT, Georgolios A, Espino S, Kaplan B, Neifeld J, et al. (2013) Percutaneous endoscopic gastrostomy site metastasis from head and neck squamous cell carcinoma: case series and literature review. J Otolaryngol Head Neck Surg 42(1): 20.
- Lee DS, Mohit-Tabatabai MA, Rush BF, Levine C (1995) Stomal seeding of head and neck cancer by percutaneous endoscopic gastrostomy tube placement. Ann Surg Oncol 2(2): 170-173.
- Adelson RT, Ducic Y (2005) Metastatic head and neck carcinoma to a percutaneous endoscopic gastrostomy site. Head Neck 27(4): 339-343.
- Lin K-T, Lin C-S, Lee S-Y, Huang W-Y, Chang W-K (2016) Risk of esophageal cancer following percutaneous endoscopic gastrostomy in head and neck cancer patients: a nationwide population-based cohort study in Taiwan. Medicine 95(9): e2958.
- Cappell MS (2007) Risk factors and risk reduction of malignant seeding of the percutaneous endoscopic gastrostomy track from pharyngoesophageal malignancy: A review of all 44 known reported cases. Am J Gastroenterol 102(6): 1307-1311.
- Mahdavi R, Faramarzi E, Mohammad-Zadeh M, Ghaeammaghami J, Jabbari MV (2007) Consequences of radiotherapy on nutritional status, dietary intake, serum zinc and copper levels in patients with gastrointestinal tract and head and neck cancer. Saudi Med J 28(3): 435440.
- Nourissat A, Bairati I, Samson E, Fortin A, Gelinas M, et al. (2010) Predictors of weight loss during radiotherapy in patients with stage I or II head and neck cancer Cancer 116(9): 2275-2283.
- Gauderer MW, Ponsky JL, Izant RJ (1980) Gastrostomy without laparotomy: a percutaneous endoscopic technique. J Pediatr Surg 15(6): 872-875.
- Cosentini EP, Sautner T, Gnant M, Winkelbauer F, Teleky B, et al. (1998) Outcomes of surgical, percutaneous endoscopic, and percutaneous radiologic gastrostomies. Arch Surg 133(10): 1076-1083.
- Moller P, Lindberg CG, Zilling T (1999) Gastrostomy by various techniques: evaluation of indications, outcome, and complications. Scand J Gastroenterol 34(10): 1050-1054.
- Mizrahi I, Garg M, Divino CM, Nguyen S (2014) Comparison of laparoscopic versus open approach to gastrostomy tubes. JSLS 18(1): 28-33.
- Miyata S, Dong F, Lebedevskiy O, Park H, Nguyen N (2017) Comparison of operative outcomes between surgical gastrostomy and percutaneous endoscopic gastrostomy in infants. J Pediatr Surg 52(9): 1416-1420.
- Tucker AT, Gourin CG, Ghegan MD, Porubsky ES, Martindale RG, et al. (2003) "Push” versus "pull” percutaneous endoscopic gastrostomy tube placement in patients with advanced head and neck cancer. Laryngoscope 113(11): 1898-1902.
- Chencharick J, Mossman K (1983) Nutritional consequences of the radiotherapy of head and neck. Cancer 51(5): 811-815.
- Beaver M (2001) Predictors of weight loss during radiation therapy Otolaryngol-Head Neck Surg 125(6):645-648.
- Ottosson S, Söderström K, Kjellen E, Nilsson P, Zackrisson B, et al. (2014) Weight and body mass index in relation to irradiated volume and to overall survival in patients with oropharyngeal cancer: a retrospective cohort study. Radiat Oncol 9(1): 160.
- Martins FP, Sousa MCB de, Ferrari AP (2011) New "introducer” PEG- gastropexy with T fasteners: a pilot study. Arq Gastroenterol 48(4): 231235.
- Cyrany J, Rejchrt S, Kopacova M, Bures J (2016) Buried bumper syndrome: A complication of percutaneous endoscopic gastrostomy. World J Gastroenterol 22(2): 618-627.
- Rahnemai-Azar AA, Rahnemaiazar AA, Naghshizadian R, Kurtz A, Farkas DT (2014) Percutaneous endoscopic gastrostomy: indications, technique, complications and management. World J Gastroenterol 20(24): 7739-7751.
- Schrag SP, Sharma R, Jaik NP, Seamon MJ, Lukaszczyk JJ, et al. (2007) Complications related to percutaneous endoscopic gastrostomy (PEG) tubes. A comprehensive clinical review. J Gastrointestin Liver Dis 16(4): 407-418.
- Hiki N, Maetani I, Suzuki Y, Washizawa N, Fukuda T, et al. (2008) Reduced risk of peristomal infection of direct percutaneous endoscopic gastrostomy in cancer patients: comparison with the pull percutaneous endoscopic gastrostomy procedure. J Am Coll Surg 207(5): 737-744.
- Lin HS, Ibrahim HZ, Kheng JW, Fee WE, Terris DJ (2001) Percutaneous endoscopic gastrostomy: strategies for prevention and management of complications. Laryngoscope 111(10): 1847-1852.
- Nevler A, Gluck I, Balint-Lahat N, Rosin D (2014) Recurrent metastatic spread to a percutaneous gastrostomy site in a patient with squamous cell carcinoma the tongue: A case report and review of the literature. J Oral Maxillofac Surg 72(4): 829-832.
- Zhang L, Dean SA, Furth EE, Weinstein GS, LiVolsi VA, et al. (2014) Metastatic carcinoma to percutaneous endoscopic gastrostomy tube sites: A report of five cases. Am J Clin Pathol 141(4): 510-514.
- Ellrichmann M, Sergeev P, Bethge J, Arlt A, Topalidis T, et al. (2013) Prospective evaluation of malignant cell seeding after percutaneous endoscopic gastrostomy in patients with oropharyngeal/esophageal cancers. Endoscopy 45(7): 526-531.
- Zamakhshary M, Jamal M, Blair GK, Murphy JJ, Webber EM, et al. (2005) Laparoscopic vs percutaneous endoscopic gastrostomy tube insertion: A new pediatric gold standard? J Pediatr Surg 40(5): 859-862.
- Russell TR, Brotman M, Norris F (1984) Percutaneous gastrostomy. A new simplified and cost-effective technique. Am J Surg 148(1): 132-137.
© 2018 Manuel Cadena, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






