- Submissions

Full Text
Open Access Biostatistics & Bioinformatics
Large Cortisol and Androgen-Secreting Adrenal Adenoma Presenting with Hirsutism: A Case Report
Ismail Engin*
Department of Endocrinology and Metabolism, University of Health Sciences, Turkey
*Corresponding author: Ismail Engin, Department of Endocrinology and Metabolism, University of Health Sciences, Umraniye Training and Research Hospital, Istanbul, Turkey
Submitted: July 09, 2025; Published: September 23, 2025

ISSN: 2578-0247 Volume4 Issue 1
Abstract
Background: Androgen-secreting adrenal tumors are a rare cause of hirsutism, accounting for less than
0.2% of cases. Most large androgen-secreting adrenal masses are malignant adrenocortical carcinomas.
We report a case of an unusually large benign adrenal adenoma simultaneously secreting cortisol and
androgens.
Case presentation: A 32-year-old woman presented with a one-year history of menstrual irregularities,
hair loss, hirsutism, and acne. Laboratory findings revealed elevated testosterone (175ng/dL), free
testosterone (7.22pg/mL), DHEA-S (1000μg/dL), and cortisol levels with failure to suppress on the
dexamethasone suppression test. Magnetic resonance imaging demonstrated an 8.5 cm left adrenal
mass with imaging characteristics initially concerning for malignancy. Laparoscopic adrenalectomy was
performed, and histopathological examination revealed a benign adrenocortical adenoma with a weiss
score of 0. The patient experienced complete resolution of symptoms postoperatively.
Conclusions: This case demonstrates that large benign adrenal adenomas can rarely present with dual
hormone secretion and significant virilization symptoms. While size and hormone profile may suggest
malignancy, histopathological examination remains the definitive diagnostic method. Complete surgical
resection provides excellent outcomes for functional adrenal adenomas.
Keywords:Adrenal adenoma; Hirsutism; Androgen excess; Cortisol; Virilization; Weiss score
Introduction
Hirsutism, defined as excessive terminal hair growth in androgen-dependent areas, affects 5-15% of women of reproductive age and represents a clinical manifestation of hyperandrogenism [1,2]. The prevalence of hirsutism varies across populations, with approximately 10% being affected in most studies [3]. While polycystic ovary syndrome accounts for 70-80% of hirsutism cases, androgen-secreting tumors represent a rare but important cause, occurring in only 0.2% of affected women [4,5]. Adrenal adenomas are benign neoplasms of the adrenal cortex that can be either hormonally active or inactive. Most adrenal incidentalomas are non-functioning adenomas discovered incidentally during imaging studies [6]. However, when functional, these adenomas typically secrete cortisol or aldosterone rather than androgens [7]. The simultaneous secretion of cortisol and androgens is more characteristic of adrenocortical carcinoma, which carries a poor prognosis [8]. Pure Androgen-Secreting Adrenal Tumors (PASATs) are exceptionally rare, with most reported cases being malignant adrenocortical carcinomas [9].
Recent systematic reviews have identified fewer than 50 cases of adult PASATs in the literature, with a predominance in women (95%) and hirsutism being the most common presenting symptom (95%) [10]. The clinical challenge lies in distinguishing benign from malignant lesions, as both can present with similar hormonal profiles and clinical manifestations. The differential diagnosis of androgen excess includes ovarian causes such as polycystic ovary syndrome and ovarian tumors, as well as adrenal causes including congenital adrenal hyperplasia, cushing syndrome, and androgen-secreting tumors [11]. The rapid onset of virilization symptoms, particularly when accompanied by significantly elevated androgen levels, should raise suspicion for an androgen-secreting tumor [12]. We present a case of a large benign adrenal adenoma simultaneously secreting cortisol and androgens, which is an extremely rare presentation that posed significant diagnostic challenges due to its size and hormonal profile.
Case Presentation
A 32-year-old woman presented to our endocrinology clinic with a one-year history of progressive menstrual irregularities, androgenic alopecia, hirsutism, and facial acne. She had no significant medical history, was not taking any medications, and had no family history of endocrine disorders. Physical examination revealed hirsutism involving the upper lip, chin, forearms, lower abdomen, and thighs, along with male-pattern baldness and facial acne. Vital signs were within normal limits. The Ferriman-Gallwey hirsutism score was 18, indicating significant hirsutism. Laboratory investigations demonstrated marked hyperandrogenism with total testosterone of 175ng/dL (normal range: 8.4-48.1ng/dL), free testosterone of 7.22pg/mL (normal range: 0-4.2pg/mL), DHEA-S of 1000μg/dL (normal range: 98.8-340μg/dL), DHEA of 1099ng/ dL (normal: <1000ng/dL), and 17-hydroxyprogesterone of 7.3 μg/L (normal range: 0.1-0.8 μg/L). Androstenedione was markedly elevated at >10μg/L (normal range: 0.51-2.1μg/L). Sex hormonebinding globulin was suppressed at 15.8 nmol/L (normal range: 30-135 nmol/L).
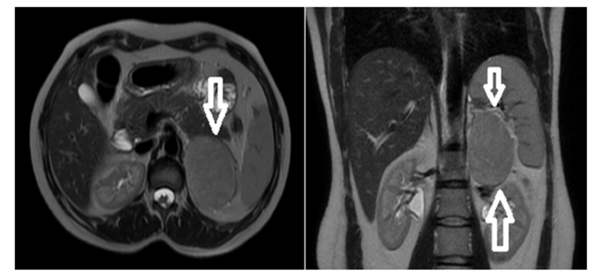
Cortisol evaluation revealed hypercortisolism with a cortisol level of 21.3μg/dL, suppressed ACTH of <1.5ng/L (normal range: 7.2-63.3ng/L), and elevated 24-hour urinary free cortisol of 562μg/24h (normal: <130μg/24h). The 1-mg dexamethasone suppression test showed failure to suppress with a cortisol level of 20.8μg/dL. Plasma metanephrine and normetanephrine levels were normal, excluding pheochromocytoma. Aldosterone and plasma renin activity were within normal limits. Given the biochemical evidence of combined hypercortisolism and hyperandrogenism, Magnetic Resonance Imaging (MRI) of the abdomen was performed. This revealed a 76×57mm well-circumscribed mass in the left adrenal gland that was isointense on both T1-weighted and T2-weighted sequences, demonstrated diffusion restriction, and showed progressive enhancement after intravenous contrast administration. The splenic vein was in close proximity to the anterior aspect of the mass, and the ipsilateral kidney showed a compression effect. Small retroperitoneal lymph nodes were noted. The imaging characteristics, combined with the large size and hormonal profile, raised concern for adrenocortical carcinoma. 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) was performed to evaluate for metastatic disease. The left adrenal mass demonstrated moderate FDG uptake with a maximum Standardized Uptake Value (SUVmax) of 7.2. No evidence of metastatic disease was identified (Figure 1).
Figure 1:Dynamic contrast-enhanced MR imaging shows a mass lesion that is isointense on T2W and T1W series with regular borders and limited diffusion, and that enhances progressively after IVCM.
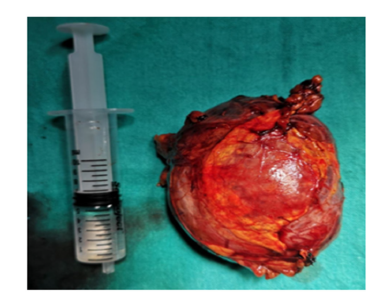
The patient underwent laparoscopic left adrenalectomy without complications. Intraoperatively, the tumor was well-encapsulated and showed no evidence of local invasion. Perioperatively, the patient received high-dose corticosteroid therapy to prevent adrenal insufficiency. Macroscopic examination revealed an 8.5×6.5×2.5 cm tumor with a firm, elastic consistency and focal soft areas. The cut surface was homogeneous yellow-orange in color, consistent with lipid-rich adrenal cortical tissue. Adjacent normal adrenal tissue was identified. Histopathological examination showed a well-circumscribed adrenocortical neoplasm without high nuclear grade, atypical mitoses, necrosis, capsular invasion, or vascular invasion. The mitotic rate was <5 per 50 high-power fields, and clear cells comprised >25% of the tumor. Immunohistochemical staining was positive for synaptophysin, inhibin, and SF-1, while chromogranin was negative. The Ki-67 proliferation index was 2%.
Based on the Weiss scoring system, the tumor scored 0 points, confirming the diagnosis of benign adrenocortical adenoma. Postoperatively, the patient’s steroid replacement was gradually tapered. At 3-month follow-up, hormonal evaluation showed normalization of androgen levels with total testosterone <2.5ng/ dL and DHEA-S of 7.1μg/dL. The 1-mg dexamethasone suppression test showed appropriate suppression with a cortisol level of 0.2μg/ dL. Clinically, the patient reported significant improvement in hirsutism, resolution of acne, and return of normal menstrual cycles. Hydrocortisone replacement therapy was gradually withdrawn, and the patient continues to do well on follow-up (Figure 2).
Figure 2:Image of the mass removed by laparoscopy.
Discussion
This case represents a rare presentation of a large benign adrenal adenoma simultaneously secreting cortisol and androgens. The combination of significant size (8.5cm), dual hormone secretion, and rapid clinical progression initially suggested adrenocortical carcinoma, highlighting the diagnostic challenges posed by such cases. Androgen-secreting adrenal tumors are exceptionally rare, with most being malignant adrenocortical carcinomas [13]. In a recent systematic review of 48 studies including 42 patients with pure androgen-secreting adrenal tumors, the authors found that malignant tumors were significantly larger (mean 8.9cm vs 4.9cm) and had shorter symptom duration (1.96 vs 4.51 years) compared to benign adenomas [10]. Our case, with its large size and relatively short symptom duration, exemplifies the diagnostic dilemma these tumors present.
The clinical presentation of hyperandrogenism in women includes hirsutism, acne, androgenic alopecia, and menstrual irregularities [14]. More severe manifestations, termed virilization, include clitoromegaly, deepening of voice, and increased muscle mass [15]. While our patient did not exhibit frank virilization, the rapid progression of symptoms and marked biochemical hyperandrogenism warranted investigation for an androgensecreting tumor. The principal adrenal androgens are DHEA, DHEA-S, and androstenedione, which serve as precursors for testosterone synthesis [16]. In our case, all these hormones were significantly elevated, consistent with adrenal androgen excess. The simultaneous elevation of cortisol with suppressed ACTH indicated autonomous cortisol secretion, a finding more commonly associated with adrenocortical carcinoma than adenoma [17].
Imaging plays a crucial role in the evaluation of adrenal masses. Unenhanced, Computed Tomography (CT) with attenuation values <10 Hounsfield Units (HU) is characteristic of lipid-rich adenomas [18]. However, approximately 30% of adenomas are lipid-poor with attenuation values >10 HU, requiring additional imaging for characterization [19]. Chemical shift MRI can detect microscopic lipids in adenomas, providing high sensitivity for adenoma diagnosis [20]. In our case, the tumor’s imaging characteristics, including progressive enhancement and diffusion restriction, were atypical for adenoma and raised concern for malignancy. The Weiss scoring system remains the gold standard for histopathological differentiation between adrenal adenomas and carcinomas [21]. The system evaluates nine parameters: nuclear grade, mitotic rate, atypical mitoses, clear cell percentage, diffuse architecture, necrosis, venous invasion, sinusoidal invasion, and capsular invasion. A score ≥3 indicates carcinoma, while scores 0-2 suggest adenoma [22]. Our case scored 0 points, definitively establishing the benign nature despite the large size and functional activity.
The 2022 WHO classification of adrenal cortical tumors emphasizes the importance of angioinvasion as a diagnostic and prognostic factor [23]. Ki-67 proliferation index >5% is associated with malignancy, though overlap exists between adenomas and carcinomas [24]. Our case demonstrated a Ki-67 index of 2%, supporting the benign diagnosis. Management of functional adrenal adenomas involves complete surgical resection, which is curative [25]. Laparoscopic adrenalectomy is the preferred approach for benign lesions, offering reduced morbidity compared to open surgery [26]. Preoperative hormonal evaluation is essential to guide perioperative management, particularly for cortisol-secreting lesions requiring steroid replacement [27]. The prognosis for benign adrenal adenomas is excellent following complete resection. In contrast, adrenocortical carcinoma carries a poor prognosis with 5-year survival rates of 40-50% [28]. This case demonstrates that even large, hormonally active adrenal masses can be benign, emphasizing the importance of histopathological examination for definitive diagnosis.
Conclusion
We report a rare case of a large benign adrenal adenoma simultaneously secreting cortisol and androgens, presenting with significant hirsutism and virilization symptoms. While the tumor’s size and hormonal profile suggested malignancy, histopathological examination confirmed its benign nature with a Weiss score of 0. This case highlights the diagnostic challenges posed by large functional adrenal adenomas and emphasizes that histopathological evaluation remains the gold standard for determining malignancy. Complete surgical resection provides excellent outcomes for functional adrenal adenomas, with resolution of hormonal excess and associated symptoms.
References
- Carmina E, Dewailly D, Norman RJ, Qiao J, Pugear M, et al. (2012) Epidemiology, diagnosis and management of hirsutism: A consensus statement by the androgen excess and polycystic ovary syndrome society. Hum Reprod Update 18(2): 146-170.
- Hohl A, Ronsoni MF, Oliveira M (2014) Hirsutism: Diagnosis and treatment. Arq Bras Endocrinol Metabol 58(2): 97-107.
- Randall VA (2008) Androgens and hair growth. Dermatol Ther 21(5): 314-328.
- Liao Z, Gao Y, Xu W, Zhao Y, Wang Z, et al. (2023) Pure androgen-secreting adrenal tumor (PASAT): A rare case report of bilateral PASATs and a systematic review. Front Endocrinol (Lausanne) 14: 1138114.
- Barbieri RL (2024) Clinical manifestations of androgen excess.
- Fassnacht M, Arlt W, Bancos I, John NP, Anju S, et al. (2016) Management of adrenal incidentalomas: European society of endocrinology clinical practice guideline in collaboration with the european network for the study of adrenal tumors. Eur J Endocrinol 175(2): G1-G34.
- Dalal A, Dwayat A, Khamashta N, Alashwas M, Asi T (2024) Resection of a pure androgen secreting adrenal adenoma in a postmenopausal woman: A case report. J Surg Case Rep 2024(1): 693.
- Else T, Kim AC, Sabolch A, Asha K, Jolly S, et al. (2014) Adrenocortical carcinoma. Endocr Rev 35(2): 282-326.
- Moreno S, Montoya G, John A, Charles P, Aubert S, et al. (2004) Profile and outcome of pure androgen-secreting adrenal tumors in women: Experience of 21 cases. Surgery 136(6): 1192-1198.
- Liao Z, Gao Y, Zhao Y, Wang Z, Jiaquan Z, et al. (2023) Pure androgen-secreting adrenal tumor (PASAT): A rare case report of bilateral PASATs and a systematic review. Front Endocrinol (Lausanne) 14: 1138114.
- Martin KA, Anderson RR, Chang RJ, David AE, Michel MP, et al. (2018) Evaluation and treatment of hirsutism in premenopausal women: An endocrine society clinical practice guideline. J Clin Endocrinol Metab 103(4): 1233-1257.
- René RG, Mario ABM, Juan MV, Eugenia TS, Azucena ZR (2013) Pure androgen-secreting adrenal adenoma associated with resistant hypertension. Case Rep Endocrinol 2013: 1-4.
- Sanja M, Dusan Z, Miladri D, Bruno C, Traini E, et al. (2024) Prevalence and clinical management of adrenal tumour-related hyperandrogenism: A narrative review. Life (Basel) 14(3): 360.
- Rosenfield RL (2005) Clinical practice. Hirsutism. N Engl J Med 353(24): 2578-2588.
- Rittmaster RS (1997) Hirsutism. Lancet 349(9046): 191-195.
- Burger HG (2002) Androgen production in women. Fertil Steril 77(suppl 4): S3-S5.
- Allolio B, Fassnacht M (2006) Clinical review: Adrenocortical carcinoma: Clinical update. J Clin Endocrinol Metab 91(6): 2027-2037.
- Rowe NE, Kumar R, Nicola S, Ferhan S, Bathini V, et al. (2023) Diagnosis, management, and follow-up of the incidentally discovered adrenal mass: CUA guideline endorsed by the AUA. J Urol 210(4): 590-599.
- Blake MA, Cronin CG, Boland GW (2010) Adrenal imaging. AJR Am J Roentgenol 194(6): 1450-1460.
- Schieda N, Alrashed A, Flood TA, Samji K, Wael S, et al. (2016) Comparison of quantitative MRI and CT washout analysis for differentiation of adrenal pheochromocytoma from adernal adenoma. AJR Am J Roentgenol 206(6): 1141-1148.
- Weiss LM (1984) Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumors. Am J Surg Pathol 8(3): 163-169.
- Mete O, Lori AE, Juhlin CC, Volante M, Papotti MG, et al. (2022) Overview of the 2022 WHO classification of adrenal cortical tumors. Endocr Pathol 33(1): 155-196.
- Juhlin CC, Mete O, Giordano TJ (2022) The 2022 WHO classification of tumours of endocrine organs: What is new in the adrenal cortex? Endocr Pathol 33(1): 47-63.
- Stojadinovic A, Brennan MF, Hoos A, Denis YL, Maria ED, et al. (2003) Adrenocortical adenoma and carcinoma: Histopathological and molecular comparative analysis. Mod Pathol 16(8): 742-751.
- Zeiger MA, Thompson GB, Duh QY, Amir HH, Julia K,et al. (2009) American association of clinical endocrinologists and american association of endocrine surgeons medical guidelines for the management of adrenal incidentalomas. Endocr Pract 15(Suppl 1): 1-20.
- Walz MK, Alesina PF, Wenger FA, Koch JA, Mann K, et al. (2006) Laparoscopic and retroperitoneoscopic treatment of pheochromocytomas and retroperitoneal paragangliomas: Results of 161 tumors in 126 patients. World J Surg 30(5): 899-908.
- Lenders JW, Duh QY, Eisenhofer G, Stefan KG, Pacak K, et al. (2014) Pheochromocytoma and paraganglioma: An endocrine society clinical practice guideline. J Clin Endocrinol Metab 99(6): 1915-1942.
- Kerkhofs TM, Verhoeven RH, Zwan JM, Jeanne D, Haak HR, et al. (2013) Adrenocortical carcinoma: A population-based study on incidence and survival in the Netherlands since 1993. Eur J Cancer 49(11): 2579-2586.
© 2025 Ismail Engin. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






