- Submissions

Full Text
Open Access Biostatistics & Bioinformatics
Maximum Oxygen Uptake Prediction Model Based on Heart Rate Variability Parameters for Young Healthy Adult Males at Rest
Wollner Materko1,2*, Rhenan Bartels1, Tiago Peçanha3, Jorge Roberto Perrout de Lima4, Alysson Roncally Silva Carvalho1,5 and Jurandir Nadal1
1 Biomedical Engineering Program (PEB), Federal University of Rio de Janeiro, Brazil
2 Laboratory of Human Movement Biodynamic, Federal University of Amapá, Brazil
3 Exercise Hemodynamic Laboratory, Universidade de São Paulo, Brazil
4 Laboratory of Motor Assessment, Universidade Federal de Juiz de Fora, Brazil
5 Laboratory of Respiration Physiology, Universidade Federal do Rio de Janeiro, Brazil
*Corresponding author:Wollner Materko, Section, Biomedical Engineering Program (PEB), COPPE Institute, Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ, Brazil and Laboratory of Human Movement Biodynamic, Physical Education, Federal University of Amapá, Brazil
Submission: August 10, 2017;Published: September 25, 2018

ISSN: 2578-0247 Volume2 Issue3
Abstract
The assessment of aerobic fitness through the measurement of maximum oxygen consumption (VO2 max) is an objective parameter that integrates cardiovascular, respiratory and metabolic responses, providing a reliable assessment of exercise capacity and health status, as well as being useful information for exercise training prescription. The purpose of this study was to determine a model for predicting Maximum Oxygen Uptake based on HRV parameters estimated at rest in 70 young physically active adults. After recording the resting tacho gram with a cardio-frequency meter to calculate HRV parameters, a maximal cardiopulmonary incremental test was performed to measure the VO2 max. The model for predicting VO2 max was obtained by stepwise multiple linear regression assuming as independent variables the mean RR interval, pNN50 index, and a proposed cardiac deceleration rate. The models were cross-validated by K-fold method, and the best model accounted for 76% of data variance, with a standard error of estimate 4.40mL·kg-1min-1. In conclusion, the obtained model might be tested as a tool for predicting the aerobic fitness in adult males in rest based on the mean RR interval and the pNN50HRV parameters. Thus, the findings are not only interesting but important in that they can be performed without the need of applying a stress test and extend the HRV applicability in the evaluation of aerobic capacity and athletic performance.
Keywords: Aerobic fitness; Cardiac deceleration rate; Heart rate variability
Introduction
The gold standard method to assess maximum oxygen consumption (VO2 max) is the maximal cardiopulmonary exercise test (CPET) [1], providing a reliable assessment of exercise capacity and health status, as well as being useful information for exercise training prescription [2]. However, CPET is not indicated for some high-risk groups, and it represents an expensive procedure due to safety requirements of medical attendance and emergency apparatus [3]. Consequently, several equations (Table 1) have been proposed to estimate maximal and submaximal oxygen uptake based on body mass, age, gender, height, perceived exertion, frequency of exercise, heart rate, walk time, run time, and load in watts [4-11]. Nevertheless, none of these studies investigated the use of HRV indexes at rest to the development of a consistent method to predict aerobic fitness.
On the other hand, the analysis of heart rate variability (HRV) is frequently used to assess the autonomic control of the heart rate fluctuations due to its wide range and cost-effective application [12-14]. It is well known that aerobic fitness promotes cardiac remodeling, leading to increased parasympathetic tone at rest [15]. Previous studies have associated HRV and VO2 max during exercise [16-18], with the objective of stratifying groups [19], in order to measure physical fitness [20-24] and muscular fatigue [25]. However, other studies based on HRV have found contradictory results, indicating that physical fitness is not associated with vascular autonomic control [26,27]. Additionally, none of these studies investigated the use of HRV parameters at rest in order to predict aerobic fitness, which is still an open field for the investigation.
Table 1:Summary of prediction models to estimate of the VO2 max for men.

Recently, Pan and colleagues [28] introduced the phaserectification signal averaged (PRSA) approach to HRV analysis that consists in separately assessing the accelerating and the decelerating phases of RR interval series in order to estimate the sympathetic and the parasympathetic contributions to heart rate control. Particularly the decelerating capacity index is being used to predict mortality after myocardial infarction [29,30] and dilated cardiomyopathy [31], to estimate physical conditioning [32,33], and to determine autonomic control status in Chagas disease patients [34]. On the other hand, it is still unknown how these parameters relate with VO2 max.
The achievement of the objectives of this study points to promising applications in the health area, when estimating aerobic conditioning the parameters derived from HRV at rest. A simple, safe, and accurate procedure for estimating the VO2 max would be a benefit to aerobic fitness prediction without the need for cardiopulmonary exercise testing using only HRV indexes at rest. Additionally, in longitudinal processes of physical conditioning, individual gains can be monitored frequently, avoiding the need for repetition of the maximum tests. The purpose of the present study was to determine and validate a model for predicting VO2 max based on parameters derived from HRV and PRSA at rest.
Material and Methodology
The sample consisted of 70 male subjects 19 to 29yrs of age. All subjects were physical education students with different levels of aerobic fitness, nonsmokers, with no history of cardiopulmonary disease, and none was taking any medication. The study protocol was approved by a local Ethical Human Research Committee of Universidade Federal de Juiz de Fora (protocol: 1230.276.2007), and an informed written consent was obtained from all subjects. This study was conducted in accordance with the instructions of the Helsinki Declaration of 2008.
Anthropometric Measurements
During an orientation session, the testing procedures and time commitment required for participation in this study were verbally explained to the potential volunteers. They were assessed for height, body mass, age, and skin fold measurement. The height was measured in centimeters while body mass was measured to the nearest 0.1kg using a mechanical scale with stadiometer (Filizola, Brazil). A skinfold caliper (Cescorf, Brazil) was used to take skinfolds measurements. Body density was estimated based on skinfolds [35] and the percent body fat was determined based on body density [36].
Experimental Procedures
The tests were conducted in a quiet room with temperature maintained at 22 °C. All subjects were instructed to avoid strenuous activity in the 24hrs prior to each testing session and to avoid alcohol, caffeine, and the consumption of large meals for at least 3hrs prior to testing. In the first visit to the lab, all subjects were instructed to lie in the supine position for 10min at rest while breathing normally. A heart rate monitor Polar RS810 (Polar, Finland) working at a sampling rate of 1000Hz was used to record RR intervals (RRi) during this period. The tachograms of RRi were transferred using an infrared interface device to the Polar Precision Performance SW software v.3.0 (Polar, Finland), which automatically corrects RRi based on moving average filter. All records of sample data showed less than 2% error and were then saved to “.txt” files.
In a second visit to the lab performed on a different day, a maximal cardiopulmonary exercise test was performed using a mechanically braked cycle ergometer 167 (Ergofit, Germany). The seat height was adjusted according to the lower limb length and the handlebars matched to the subject’s own riding position. The protocol was divided into three phases:
1. Rest-4 min seated rest.
2. Test-incremental workload until exhaustion (25W·min-1, maintaining 50 to 60rev·min-1).
3. Recovery-15min recovery, the first 3min consisting of active cycling (12.5W) followed by a 12-min passive rest.
Throughout the tests, the pulmonary gas exchange variables were determined breath-by-breath using a VO2 OOO metabolic analyzer (Med Graphics, EUA), which was calibrated in automatic mode before each test. The oxygen consumption and other variables were continuously drawn from the facemask connection that was sampled at intervals of 20sec. The aerobic fitness of the subjects was expressed by the values of VO2 max at peak conditions. For safety concerns, electrocardiogram was monitored during the test using a multi-parametric monitor (Dixtal, Brazil).
Data Analysis
The HRV analysis in the time domain was performed by the Sinus Cor [37] Matlab package software (Pulmonary Engineering, PEB/COPPE/UFRJ, Brazil) to obtain the classical parameters
1. pNN50 - the percentage of times in which the change in consecutive normal sinus (NN) intervals exceeded 50ms.
2. SDNN-The standard deviation (SD) of all NN intervals (SDNN).
3. RMSSD-The root mean square of SD between adjacent NN intervals.<./
4. Mean RRi-The mean of NN intervals. The RRi series was interpolated by cubic splines and resampled with a frequency of 4Hz to obtain equally sampled signal.
The spectral analysis was then performed using the Welch Periodogram Method (segments of 256 points with 128 points of overlap using Hanning window), to obtain the spectral indexes:
1. LF - low-frequency (0.04-0.15Hz).
2. HF - high-frequency (0.15-0.40Hz).
3. LF/HF ratio. All parameters were computed as recommended by the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology [38].
Following the original proposal of Bauer et al. [30], the decreasing and increasing phases of heart rate were also analyzed separately, in order to better estimate the contributions of the parasympathetic and sympathetic control, respectively. In the present study, a simplification was proposed. First, a vector was created of the differences between successive elements of RRi series and, then, the Cardiac-Deceleration Rate (CDR) was defined as the mean of the positive values, and the Cardiac Acceleration Rate (CAR) as the mean of the negative values. All signal analysis procedures were performed with programs written in Matlab version 6.5 (The Math Works, USA).
Statistical Analyses
Descriptive statistical analyses of the data were expressed as mean±standard deviation and 95% confidence interval. The Kolmogorov-Smirnov test confirmed the normality of distribution and the size effect of the sample was considered large with actual power of 0.99. The correlation between all parameters (HRV and PRSA) VO2 max were tested by the Pearson correlation test. The experimental model for predicting VO2 max was obtained through the stepwise multiple linear regression technique set for both forward and backward directions, using the Akaike Information Criterion (AIC), and assuming as independent variables the HRV parameters, including CDR and CAR indexes. The model accuracy was determined by the adjusted R2 value and the Standard Error of the Estimate (SEE) between the measured and predicted VO2 max. The SEE was calculated as SDy/(1-R2), where SDy is the standard deviation of the measured VO2 max and R2 is the coefficient of determination. The model obtained was cross-validated by K-fold method [39] with K=5 and N=70. The sample was randomly divided into five data sets and model estimation was repeated five times, taking 56 subjects data for training the other 14 for test set. Each test set was composed of 14 randomly selected subjects, being seven above and seven below the median VO2 max. Each resulting model consisted of a free intercept and a different coefficient for each variable selected during the respective fold. Thus, it was tested to predict VO2 max values of each case in the respective independent test set, which were compared to the measured ones. Finally, the five partial results were combined to obtain the resulting adjusted R2 and SEE values.
For generalized results, the best model was applied in 70 subjects. The measured and estimated values for VO2 max were correlated and the model reliability was expressed by analyzing the residues. All procedures assumed P≤0.05 for statistical significance and were processed in the R statistical software v.3.3.1 and Mat lab v6.5 (Math works, EUA).
Result
Anthropometric and physical characteristic of the subjects were very similar. They presented low SD values and confidence intervals with high precision (Table 2). The correlation values between the independent parameters and VO2 max are summarized in Table 3, which shows significant linear associations between VO2 max and Mean RRi, RMSSD, pNN50, and CDR and CAR parameters. After excluding no significant co-variables, all five models included Mean RRi, CDR and pNN50 parameters (Table 4). The adjusted R2 and SEE values were similar, ranging from 0.69 to 0.76 and from 4.34 to 4.74, respectively, with average values 0.72±0.02 and 4.49±0.15, respectively. As all models showed adequate prediction capabilities, including exactly the same variables. The best model according to R2 (Fold #1) was chosen and applied to predict the VO2 max of all 70 subjects. Considering this model, all the added variables have significant additional contribution (Table 5) that increases R2 and decreases SEE, thus increasing the predictive power of the model.
Table 2:Physical and anthropometric characteristics of participants.

where SD is standard deviation
Table 3:Correlations between independent parameters and VO2 max.

*Significant difference in t-test
Table 4:Cross validation for five models to estimate of the VO2 max.

Mean RRi is the mean of NN intervals; CDR: Cardiac Deceleration Rate; pNN50 is the percentage of times in which the change in NN intervals exceeds 50ms
Table 5:Cumulative R2, standard error of the estimate and p values of the parameters added to the selected model to estimate VO2 max.

Mean RRi is the mean of NN intervals; CDR: Cardiac Deceleration Rate; pNN50 is the percentage of times in which the change in NN intervals exceeds 50ms
Figure 1:
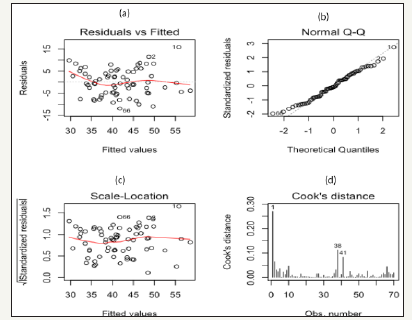
The best model explained 76% of original data variance, with a standard error of estimate of 4.40mL·kg-1·min-1. The average measured VO2 max (41.6±8.2mL·kg-1·min-1) and predicted VO2 max (41.9±8.2mL·kg-1min-1) values showed no significant difference (P>0.01) 0.34±4.43mL·kg-1·min-1. The measured and predicted VO2 max values obtained for all 70 subjects presented a good correlation (r=0.87, P>0.01). The reliability of the regression model was studied by analyzing the residues (Figure 1) in which it is observed that the data dispersion does not present an apparent pattern (Figure 1), which characterizes the homogeneity of the variance since the data are close to zero (Figure 1) showing the linearity of the data (Figure 1). Furthermore, in the Cook Distance graph (Figure 1) illustrates the presence of few outliers (subjects 1, 38, and 41).
Discussion
The purpose of this study was at predict the maximum oxygen uptake of young healthy male subjects by multiple linear regression based on parameters derived from the resting heart rate variability. The linear regression model was successfully fitted to the subjects’ data, and the model parameters are expected to be related to aerobic fitness. Previous studies [15,40-43] have associated HRV and aerobic fitness. High aerobic fitness subjects trend to present higher values of HF power and increase in time domain parameters of the HRV related to parasympathetic activity. In accordance, all three indexes included in the model proposed in the present study are also related to parasympathetic activity as follows.
Current findings indicate that subjects with higher values of Mean RRi are associated with high aerobic fitness. Several investigations have studied the mechanisms responsible for the resting bradycardia in subjects with high aerobic fitness [41,44]. Changes in the intrinsic mechanisms acting on the sinus node and alterations in the autonomic nervous system control of the heart have been reported to contribute to this phenomenon [45,46]. These findings lead to the hypothesis that high aerobic conditioning is related to high cardiac efficiency with an improved ejection fraction. In this condition, lower heart rates (due to the increase in vagal activity) are required to maintain the arterial blood pressure. To better investigate this hypothesis, the phase rectified HRV analysis was performed.
In the original PRSA method proposed by Bauer et al. [30], each positive change of RR time series was used as the anchor for the coherent average of the surrounding RR interval segment, and the resulting step was defined as the decelerating capacity index. Similarly, the accelerating capacity index corresponded to the respective step obtained from the negative RR changes. Nasario et al. [32-33] proposed a change in this method, by considering as anchor for the coherent average only the steepest change in each period of positive or negative changes of RR time series. This approach was successful for stratifying athletes with high aerobic fitness from normal subjects.
In the present study, the proposal was to simplify the method by avoiding the calculus of coherent averages, since the only interest was on the height of the resulting step. The mean value of positive changes was taken as a cardiac deceleration rate. Therefore, as the occurrence of lower resting heart rate was associated with high aerobic fitness in some subjects, it was assumed that they were prone to higher vagal tonus, and thus are able to present higher values of CDR, as observed. The result of the linear regression model supports the hypothesis that CDR adequately represented the parasympathetic control of the subjects’ heart rate, and thus are appropriate in estimating the positive adaptations that associated with a higher level of cardiorespiratory fitness [20- 24], or the autonomic control of post-exercise heart rate [40-46] and the increase in vagal activity [47-49]. Thus, it is reasonable to speculate that regular aerobic exercise, with a sufficient intensity to cause an increase in VO2 max could be evaluated by the CDR that express changes in heart rate mainly due to parasympathetic control [15,40,41]. Additionally, the pNN50 is also associated with increased parasympathetic modulation, since it is directly related to aerobic fitness [42,43].
The need to assess aerobic capacity in the general public has led to the development of various exercise and non-exercise prediction models. Several studies have developed submaximal run test in order to predict the VO2 max based on 1.5 mile run [8], Rockport walking test [11], the 1-mile Jog test [8], Single-stage submaximal treadmill test [10], non-exercise tests [6,7,9] or the use of both exercise and non-exercise data [4,5]. The SEE and R2 values in these models ranged from 3.0 to 5.64mL·kg-1·min-1 and 0.66 to 0.88, respectively. Thus, the model proposed in the present study, using resting parameters, showed the lowest SEE value (4.40mL·kg- 1·min-1) of all models based on resting data, which is comparable to the models based on the submaximal tests. Such models are effective for use in large epidemiological cohorts in which an exercise tests to predict or measure VO2 max would be impractical.
To the extent of our knowledge, the presented model is the first to predict aerobic fitness from parameters derived only from heart rate variability at rest. It was validated with a homogeneous sample of young (22.0±2.6yrs) healthy male subjects, which is characterized as physically active college students, but not athletes, since the subjects presented a limited range of VO2 max values with levels of aerobic fitness (41.6±8.2mL·kg-1·min-1) near the population mean of 40mL·kg-1·min-1 for young people [50]. The correlation obtained between estimated and measured values for such a uniform sample is suggestive of applicability to larger epidemiological cohorts. However, the sample characteristics could be viewed as a limitation, and the potential application to other samples, including both genders and subjects who could not be submitted to a maximal cardiopulmonary test should be investigated.
Conclusion
A model based on the mean RR interval at rest and the pNN50 HRV parameters and a novel cardiac deceleration rate was proposed and validated to predict the VO2 max in young healthy adult male subjects. These results emphasize the importance of evaluating heart rate variability at rest and the potential to estimate the physical performance of subjects without the need for a maximum or submaximal test.
Acknowledgement
This work was partially supported by the Brazilian Research Council (CNPq) and CAPES Foundation. Conflict of Interest Statement. The authors declare that the research was conducted in the absence of any commercial or financial relations hips that could be construed as a potential conflict of interest.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relations hips that could be construed as a potential conflict of interest.
References
- Wasserman K, Whipp BJ (1975) Exercise physiology in health and disease. Am Rev Respir Dis 112(2): 219-249.
- Wasserman K, Hansen, JE, Sue DY, Stringer WW, Whipp BJ (2005) Principles of exercise testing and interpretation: including pathophysiology and clinical applications. Medicine & Science in Sports & Exercise 37(7): 1249.
- Poole DC, Jones AM (2012) Oxygen uptake kinetics. Comprehensive Physiology 2: 933‐996.
- Nielson DE, George JD, Vehrs PR, Hager RL, Webb CV (2010) Predicting VO2 max in college-aged participants using cycle ergometry and perceived functional ability. Measurement in Physical Education and Exercise Science 14: 252-264.
- George JD, Bradshaw DI, Hyde A, Vehrs PR, Hager RL, et al. (2007) A maximal graded exercise test to accurately predict VO2max in 18-65-year-old adults. Measurement in Physical Education and Exercise Science 11: 149-160.
- George JD, Stone WJ, Burkett LN (1999) Non-exercise VO2max estimation for physically active college students. Med Sci Sports Exerc 29: 415-423.
- Heil DP, Freedson PS, Ahlquist LE, Price J, Rippe JM (1995) Non exercise regression models to estimate peak oxygen consumption. Med Sci Sports & Exerc 27(4): 599-606.
- George JD, Vehrs PR, Allsen PE, Fellingham GW, Fisher AG (1993) VO2max estimation from a submaximal 1-mile track jog for fit collegeage individuals. Med Sci Sports Exerc 25(3): 401-406.
- Ainsworth BE, Richardson MT, Jacobs DR, Leon AS (1992) Prediction of cardiorespiratory fitness using physical activity questionnaire data. Medicine Exercise Nutrition and Health 1: 75-82.
- Ebbeling CB, Ward A, Puleo EM, Widrick J, Rippe JM (1991) Development of a single-stage submaximal treadmill walking test. Med Sci Sports Exerc 23(8): 966-973
- Kline GM, Porcari JP, Hintermeister R, Freedson PS, Ward A, et al. (1987) Estimation of VO2 max from a one-mile track walk, gender, age, and body weight. Med Sci Sports Exerc 19: 253-259.
- Billman GE, Huikuri H, Sacha J, Trimmel K (2015) An introduction to heart variability: Methodological considerations and clinical applications. Front Physiol 6: 55.
- Billman GE (2013) The effect of heart rate on the heart rate variability response to autonomic interventions. Front Physiol 4: 222
- Billman GE (2011) Heart rate variability a historical perspective. Front Physiol 2: 86.
- Buchheit M, Gindre C (2006) Cardiac parasympathetic regulation: Respective associations with cardiorespiratory fitness and training load. Am J Physiol Heart Circ Physiol 291(1): H451-H458.
- Melanson EL, Freedson PS (2001) The effect of endurance training on resting heart rate variability in sedentary adult males. Eur J Appl Physiol 85(5): 442-449.
- Rosenwinkel ET, Bloomfield DM, Arwady MA, Goldsmith RL (2001) Exercise and autonomic function in health and cardiovascular disease. Cardiol Clin 19(3): 369-87.
- Kawaguchi LYA, Nascimento ACP, Lima MS, Frigo, L, Paula AR, et al. (2007) Characterization of heart rate variability and baroreflex sensitivy in sedentary individuals and male athletes. Revista Brasileira de Medicina do Esporte 13: 207-12.
- Marocolo M, Nadal J, Barbosa PR (2007) The effect of an aerobic training program on the electrical remodeling of the heart: High-frequency components of the signal-averaged electro-cardiogram are predictors of the maximal aerobic power. Braz J Med Biol Res 40: 199-208.
- Tonello L, Reichert FF, Oliveira Silva I, Del Rosso S, Leicht AS, et al. (2015) Correlates of heart rate measures with incidental physical activity and cardiorespiratory fitness in overweight female workers. Front Physiol 6: 405.
- Silva DA, Cardew A, Qasem L, Wilson RP, Lewis MJ (2015) Relationships between oxygen uptake, dynamic body acceleration and heart rate in humans. J Sports Med Phys Fitness 55(10): 1049-57.
- Kaikkonen KM, Korpelainen RI, Tulppo MP, Kaikkonen HS, Vanhala ML, et al. (2014) Physical activity and aerobic fitness are positively associated with heart rate variability in obese adults. J Phys Act Health 11(8): 1614-1621.
- Da Silva DF, Verri SM, Nakamura FY (2013) Longitudinal changes in cardiac autonomic function and aerobic fitness indices in endurance runners: A case study with a high-level team. Eur J Sport Sci 14(5): 443- 451.
- Aslani A, Aslani A, Kheirkhah J, Sobhani V (2011) Cardio-pulmonary fitness test by ultra-short heart rate variability. J Cardiovasc Dis Res 2(4): 233-236.
- Wallace LK, Slattery KM, Coutts AJ (2014) A comparison of methods for quantifying training load: relationships between modeled and actual training responses. Eur J Appl Physiol 114(1): 11-20.
- Grant CC, Murray C, Janse Van Rensburg DC, Fletcher L (2013) A comparison between heart rate and heart rate variability as indicators of cardiac health and fitness. Front Physiol 4: 337.
- Bosquet L, Gamelin FX, Berthoin S (2007) Is aerobic endurance a determinant of cardiac autonomic regulation? Eur J Appl Physiol 100(3):363-369.
- Pan Q, Zhou G, Wang R, Cai G, Yan J, et al. (2016) Do the deceleration/ acceleration capacities of heart rate reflect cardiac sympathetic or vagal activity? A model study. Med Biol Eng Comput 54(12): 1921-1933.
- Rizas KD, Eick C, Doller AJ, Hamm W, von Stuelpnagel L, et al. (2017) Bedside autonomic risk stratification after myocardial infarction by means of short-term deceleration capacity of heart rate. Europace 20(FI1): f129-f136.
- Bauer A, Kantelhardt JW, Barthel P, Schneider R, Mäkikallio T, et al. (2006) Deceleration capacity of heart rate as a predictor of mortality after myocardial infarction: cohort study. Lancet 367(9523): 1674-1681.
- Zou C, Dong H, Wang F, Gao M, Huang X, et al. (2016) Heart acceleration and deceleration capacities associated with dilated cardiomyopathy. Eur J Clin Invest 46(4): 312-320.
- Nasario Junior O, Benchimol Barbosa PR, Pedrosa RC, Nadal J (2014) Refining the deceleration capacity index in phase-rectified signal averaging to assess physical conditioning level. J Electrocardiol 47(3): 306-310.
- Nasario Junior O, Benchimol Barbosa PR, Pedrosa RC, Nadal J (2015)
Beat-to-beat ventricular repolarization duration variability assessed by
cardiac acceleration and deceleration phases in athletes. EC Cardiology
1: 33-42.
- Nasario Junior O, Benchimol Barbosa PR, Pedrosa RC, Nadal J (2015) Assessment of autonomic function by phase rectification of RR-Interval histogram analysis in Chagas disease. Arq Bras Cardiol 104(6): 450-456.
- Jackson AS, Pollock M (1978) Generalized equations for predicting body density of men. Br J Nutr 40(3): 497-504.
- Siri WE (1961) Body composition from fluid spaces and density: analysis of methods, apud: Brozek, J and Henschel. Techniques for measuring body composition. Washington National Academic of Science, USA.
- Bartels R, Neumamm L, Peçanha T, Carvalho ARS (2017) SinusCor: An advanced tool for heart rate variability analysis. Biomedical Engineering Online 16(1): 110.
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (1996) Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation Research 93: 1043-1065.
- Rodríguez JD, Pérez A, Lozano JA (2010) Sensivity analysis of K-fold Cross validation in prediction error estimation. IEEE Transactions on Pattern Analysis and Machine Intelligence 32(3): 569-575.
- Moreira DN, Peçanha T, Guerra ZF, Silva LP, Nakamura FY, et al. (2013) Effects of aerobic fitness on cardiac autonomic modulation during supine and upright posture. Journal of Exercise Physiology Online 16: 92-100.
- Buchheit M, Chivot A, Parouty J, Mercier D, Al Haddad H, et al. (2010) Monitoring endurance running performance using cardiac parasympathetic function. Eur J Appl Physiol 108(6): 1153-1167.
- Yataco AR, Fleisher LA, Katzel LI (1997) Heart rate variability and cardiovascular fitness in senior athletes. Am J Cardiol 80(10): 1389- 1391.
- De Meersman RE (1993) Heart rate variability and aerobic fitness. Am Heart J 125(3): 726-731.
- Plews DJ, Laursen PB, Stanley J, Kilding AE, Bucheit M (2013) Training adaptation and heart rate variability in elite endurance athletes: opening the door to effective monitoring. Sports Med 43(9): 773-781.
- Martinelli FS, Chacon Mikahil MPT, Martins LEB, Lima Filho EC, Golfetti R, et al. (2005) Heart rate variability in athletes and non-athletes at rest and during head-up tilt. Braz J Med Biol Res 38(4): 639-647.
- Carter JB, Banister EW, Blaber AP (2003) Effect of endurance exercise on autonomic control of heart rate. Sports Med 33(1): 33-46.
- Carnevali L, Sgoifo A (2014) Vagal modulation of resting heart rate in rats: the role of stress, psychosocial factors, and physical exercise. Front Physiol 5: 1-12.
- Peçanha T, de Paula Ribeiro M, Nasario Junior O, de Lima JR (2013) Postexercise heart rate variability recovery: a time-frequency analysis. Acta Cardiologica 68(6): 607-613.
- Trevizani GA, Belchimol Brbosa PR, Nadal J (2012) Effects of age and aerobic fitness on heart rate recovery in adult men. Arq Bras Cardiol 99(3): 802-810.
- Thompson WR (2014) American college of sports medicine acsm’s guidelines for exercise testing and prescription 8th Edition. Lippincott Williams & Wilkins, Philadelphia, USA.
© 2018 Wollner Materko. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






