- Submissions

Full Text
Novel Techniques in Nutrition and Food Science
Milk Casein Types, Human Health and Probiotic Bacteria
Necibe Canan USTA*
Biology Department, Art & Science Faculty, Gaziosmanpasa University, Turkiye
*Corresponding author:Necibe Canan USTA, Biology Department, Art and Science Faculty, Gaziosmanpasa University, Turkiye
Submission: September 15, 2025;Published: November 06, 2025

ISSN:2640-9208Volume8 Issue 4
Abstract
There have globally been 30-40fold increases in the prevalence of some chronic diseases like cardiovascular disease, type 1 diabets, undiagnosed gastrointestinal conditions, some disordered nervous, endocrine and immune system diseases. Recently, a relationship between disease risk and consumption of a specific bovine β-casein fraction, A1 or A2 genetic variants, has been identified. Populations consuming milk containing high levels of β-casein A1 variant, due to the milk-derived peptides, β-casomorphins (BCMs), have a higher incidence of type 1 diabetes, severe symptoms of autism and schizophrenia, endocrine, immune system, allergys and gastrointestinal diseases. It was observed in the studies that, BCM7 permeates the intestinal brush border barrier and may induce several biological effects through the μ-opioid receptors (MORs). The mechanism of action generally begins with β-casein A1 and its related forms that produce a bioactive opioid peptide, β-casomorphin-7 (β-CM-7) during digestion. On the other hand, the researchers have postulated that patients with those systemic disorders drive clinical benefit when consume cow milk having A2 allelic variant. New adjustments to the non-hybrid animal milk supply can be readily made, indeed. In addition, actually, Bifidobacteria and lactic acid bacteria, are the ones emphasized as effective approaches. Intestinal bacterial flora replacement can be used to bypass the negative casein effects in the gut, whether by natural food consumption or in a supplementary diet. The probiotic bacterial species have capability of enzymatic hydrolysis of A1 casein derived β-casomorphins, (BCMs) molecules. On the other hand, every step investigating the role of milk casein types and Further research would always be needed for new probiotic usage and preventive measures, for blocking its adverse effects.
Graphical Abstract
Figure 1:
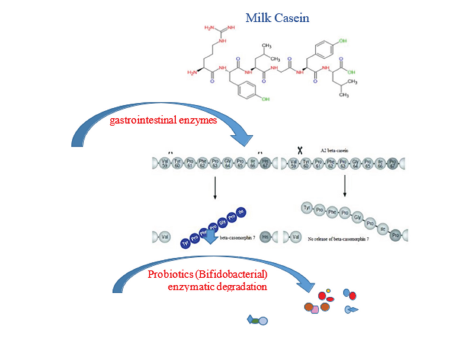
Highlights
a) Bovine milk casein types differ with animal breeds.
b) βeta A1 casein proteins have been related to some systemic diseases in human.
c) The amino acid shift, (prolin to histidine at residue 67) in the mature A1 casein protein yielding bioactive β-casomorphin-7, β-CM-7, was reported
to play role in some systemic disfunctions.
d) Generally local breeds (high interior genetic homogeneity) contain more A2 allelic casein in their milk.
e) Some Lactobacillus spp and Bifidobacteria spp seem to prevent β-casomorphin-7 protein activities in the human gut in some ways.
Introduction
Milk is a convenient source of general nutrition for physiological requirements and cattle (cow, taurus) milk has been the most consumed milk as a worldwide. As geographical climate changed, the milk source also has changed with as a non-cattle (goat, sheep, buffalo) milk in human diet since ancient times [1]. The biosynthesis of animal milks components differs in carbohydrates, fatty acids, proteins, minerals dissolved gases, enzymes, vitamins and other milk soluble. They change within breed. In addition, their environment, diet, totally the management applications determine the milk composition. Genetic variation within breed results in changes in the animal mammary gland biosynthesis that is, secretion of the milk components.
Carbohydrates in milk
The predominant carbohydrate in milk is the disaccharide lactose, composed of one molecule of galactose and one molecule of glucose, also named as beta-galactoside (1-4 carbon linkage), hydrolised by β- galactosidase. Lactose is the substrate of Lactobacillus spp. bacterial fermentation. Lactose in milk also regulates water content in osmosis [2]. The other part of carbohydrates are monosaccharides, sugar phosphates, nucleotide sugars, free neutral and acid oligosaccharides and glycosyl groups of peptides and proteins. Free glucose and galactose and the sugar alcohol myo-inositol are also present in milk. However, the amounts of these carbohydrate fractions are minor compared with that of lactose [3].
Milk lipids
Fatty acids are another energy molecules in the milk. The predominant fat in milk is triacylglycerol, which contains fatty acids of short-(C4-C10), intermediate-(C12-C16), or long-chain (C18) length. Fat, indeed, is the main source of energy found in the milk, especially, goat and cow milk are low in polyunsaturated fatty acids that are necessary for human metabolism [4]. The lipid composition of milk includes two major groups: simple lipids (triglycerides) and complex lipids (phospholipids). The milk fat has a nutritional role by energy intake, 9kcal/g as dietary lipids. Major components being triglycerides and phospholipids (mainly phosphatidylcholine, sphingomyelin, phosphatidylethanolamine, phosphatidylserine and phosphatidylinositol [5].
Milk proteins
The milk proteins are several types; wheys and caseins; alpha, beta and kappa casein, composing of composed of different amino acids ranging in number from 196 to 203. Further, the type of amino acids also depends on the source of milk, the animals, so it is difficult to get its exact chemical formula. It is found in primary structure composed of amino acids. It does not have any secondary and tertiary structure like globular protein. The caseins are found in the milk in a miceller form, getting together by naturally suggested peptide interactions along with calcium phosphate bridges. The protein part of the milk (total crude protein content) is determined by analyzing milk for nitrogen, so that not only casein part but also non protein nitrogen molecules account the nitrogen content, the whey part, (Figure 1).
Figure 2:Caseins and whey protein fractions in total milk protein [48].
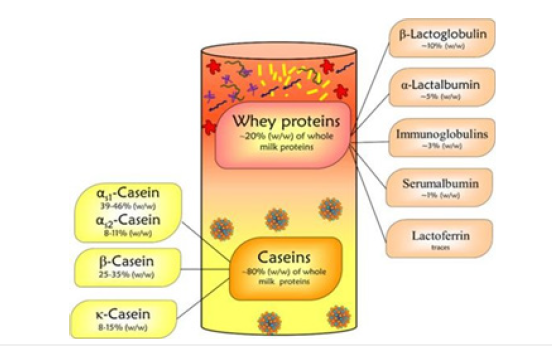
Animal casein proteins generally are characterized by high proline amino acid contents, ester-bound phosphate and few cysteine residues. The main casein types in milk are alpha-(alpha S-1, alpha S-2), beta-, gamma-and kappa-caseins. The gamma casein is the hydrolised part of the beta casein when the milk kept at 40C. The hydrophobic gamma-casein is formed by proteolytic degradation of beta-casein.
In Figure 2, the micelle image obtained by red-blue 3-dimensional glasses to visualize the electron-dense locations (i.e., the dark pixels) as individual entities ranging in size from 1×1.5 to 4×4nm. This is the tertiary structure of the caseins, such that some portions of the peptide molecules would be compact (and therefore electron-dense) and other portions would be more open (and therefore less electron-dense). Those regions of the peptides that carry negative charges would also bind the uranyl ion more, so the hydrophobic regions devoid of negatively charged side groups would be less heavily stained, so, only portions of the casein molecules were being imaged. The casein micelle includes different plasmin enzymes, salt components, calcium and phosphorus salts, embedded lipase, casein proteins and whey in varying acidic pH approximetely 4.0. Their proportions differ depending on the general milk composition in turn depends animal feeding end other environmental conditions, other than main breed. Practically there seem four main ways to induce aggregation by the use of proteolytic enzymes or in acidic conditions with heat treatment. The final gelation continues by aging.
Figure 3:Anaglyphic version of transmission electron micrographs of (A) freeze-dried immobilized and uranyl oxalatestained casein micelle, (B) a digitally magnified peripheral region of a casein micelle [44].
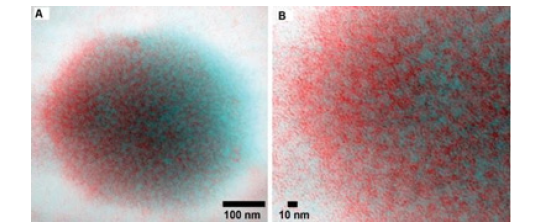
The self-assembled casein forms which are made up of hundreds of individual amino acids, each of which may have a positive or a negative charge, depending on the pH of the milk system. At some pH value, all the positive charges and all the negative charges on the casein remain in balance, that is the resulting protein is neutral and this state is named the isoelectric point (4.6 for casein). At this point the proteins are least soluble. Milk has a pH value of about 6.6, at which the casein micelles have a net negative charge and are quite stable [6] (Figure 3).
Figure 4:The chemical structure of casein protein (Bounous et al. 1981).
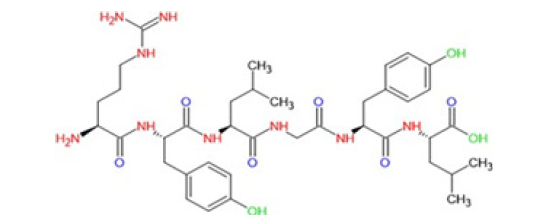
Salt molecules bind with the casein peptides and make aggregation named micelle, the curd structure. In the presence of Ca2+ Alpha and β casein proteins are coagulated. The remaining proteins in solution forms whey, along with the lactose and salts. This micellars coagulate and polymerize to form a curd and this expels the whey by syneresis (separation of water component from coagulated casein micells). This process is the traditional cheese making way in many countries, indeed [7]. Indeed, more than 95% of the proteins in the milk of goat and other livestock are encoded by casein and whey protein genes [8]. There are variations in animal milk casein types. They differ in their amino acid sequences. related to the type of polypeptide chains, beta-lactoglobulin and alpha-lacto-albumin with casein [9]. Milk casein protein molecules are highly diverse within and across animal species. From ancient times, the genes were the most divergent ones in the milk [10]. The differences in amino acids across species were explained as most probably the shuffling of the coding portions of the genes, αs1- and αs2-caseins, as a result of the mRNA post modification in the nucleus of the cells, so the exons reassembled. In contrast, point mutations has played a role in β-casein variation, an amino acid exchange in DNA code [11] (Figure 4).
Figure 5:A neighbor-joining tree representing the phylogenetic analysis of casein subunits (alpha-S1-, alpha- S2-, beta-, and kappa-casein amino acid sequences) from different domestic animals, sheep, goats, cattle, Arabian camels, water buffalos and rats. (Sensu Tomasz Konrad et al, 2017), αs1, αS1-casein; αs2, αS2-casein; β, β-casein; κ, κ-casein [22].
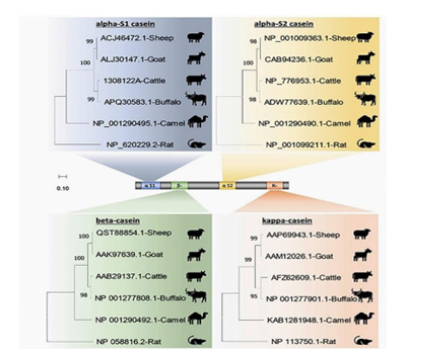
(The tree was rooted in Rattus norvegicus. Bootstrap support values are indicated on the nodes. Bootstrap values were calculated from 1,000 replications) (Figure 5).
Figure 6:Model of the casein micelle [49].
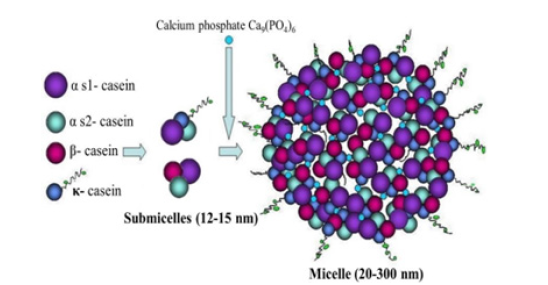
Genetic variants for the milk protein groups have been demonstrated by Gaunt and breed differences have been found for the frequency of occurrence of these variants [12]. In the preliminary studies, it had been known that caseins proteins (αs1, β, αs2 and κ) in ruminant animals are coded by single-copy genes, in a region of approximately 300 kb, physically linked in the following order: CSN1S1 (αs1-Cn), CSN1S2 (αs2-Cn), CSN2 (β-Cn) and CSN3 (κ-Cn) [13]. Later after ongoing researches, it was determined that the casein protein complex includes four caseins, namely, alpha S1, alpha S2, beta and kappa caseins. The casein amino acid sequence differs in these proteins and stated that, they were encoded by four casein genes (CSN1S1, CSN1S2, CSN2 and CSN3) on a cluster on chromosome 6 [11].
Interestingly, β-casein in goat milk exists mainly as the A2 type [14] and it does not produce β-casomorphin-7 (BCM-7), generated during the process of milk digestion, which may be related with various disorders, such as gastrointestinal disturbances.
Milk digestion
Digestional products of the curd structure from coagulated milk have some differences due to not only protein composition but also digesting conditions. There are different physiological processing conditions in the stomach, especially the temperature and time together may induce differences in the final curd structure, which in turn influence the rate of delivery of solubilized proteins to the small intestine and their subsequent reabsorption. This is wholly the dynamic gastric digestion, the behavioral movement of skim milk (raw and heated 90 °C, 20min) (Figure 6).
Figure 7:Diagram of the model mechanism of the steps during the protein curd formation; (A) raw milk, (B) heated milk, in gamicell formation, while in the heated cattle milk stric digestion [50].
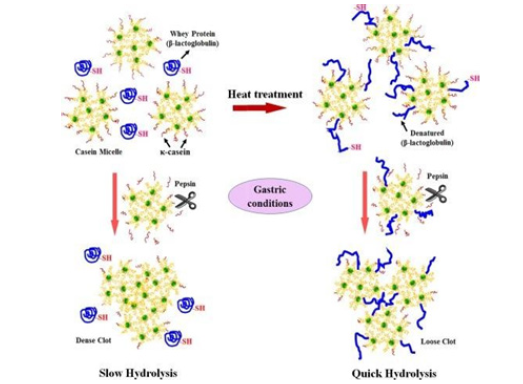
In another study, it was demonstrated that, raw milk curdling resulted only in casein micelles while in heated milk coagulation denatured whey proteins formed in addition to casein micelles in coagulation processes. In that study, heating (90 °C for 20min) would have also led to complex aggregations between denatured whey proteins and casein peptides in the presence of sulfhydryl groups and so the disulfide bridges [15].
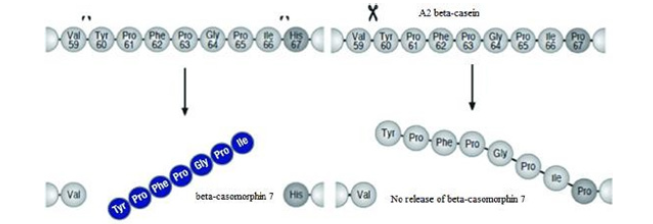
Recently, there are two general families of beta-casein proteins, known as A1 and A2 beta- casein types. [16]. According to the recent studies, the A1 type variant, herds, has arosen from the original A2 type, most probably~5000-10 000 years ago in European area. It was demonstrated that a Proline 67 to Histidine 67point mutation had occurred in the past.3Incountries that have dairy cows of northern European ancestry, the relative proportions of the co-dominant A1 to A2 beta-casei nalleles are typically 1:1 in cows, which then produce the same ratio of A1 to A2 beta-casein in milk. This tends to be lower in breeds from Southern Europe. However, this ratio depends on the specific breeding history of the dominant breeds. Once milk or milk products are consumed, the action of digestive enzymes in the gut on A1 beta-casein releases the bioactive opioid peptide beta-casomorphin-7 (BCM-7);4-8in contrast, A2 beta-casein releases much less and probably minimal amounts of BCM-7 under normal gut conditions.7-10BCM-7 is a mu-opioid receptor ligand,8,11andmu-opioid receptors are expressed widely throughout human physiology, including the gastrointestinal tract
Digestional diseases of milk
Recently, a relationship between disease risk and consumption of a specific bovine ss-casein fraction either A1 or A2 genetic variants has been identified. Populations, which consume milk containing high levels of ss-casein A2 variant, have a lower incidence of cardiovascular disease and type 1 diabetes. Whether there is a definite health benefit to milk containing the A2 genetic variant is unknown and requires further investigation. β-casomorphin-7 (BCM7) has been related with many disorders in human health. Consumption of milk with the A2 variant may be associated with less severe symptoms of autism and schizophrenia.
In recent years, the role of the opioid system in physiology various pathologies have been attracking the attention of researchers. Numerous studies confirmed the penetration of milk/casein-disolved opioid peptides through intestinal barrier and induce systemic effects on the opioid receptors of the cells in nervous system, in turn the immune responses etc.… [17]. Milk derived peptides like opioids from hydrolysed bovine milk on DPPIV gene expression in peripheral blood mononuclear cells in autistic and healthy children were determined using the Real- Time PCR (Polymerase Chain Reaction) method [18]. Results of some related studies demonstrated hyperleptinemia followed by increased blood– brain barrier permeability that in turn causing accumulation of beta casomorphin BCM7 in the blood and the brain, leading to the development of autism spectrum disorders. In addition, it has been suggested that peptiduria in autistic children is the potential defect in their proteolytic excretion systems. Infants with delayed psychomotor development showed elevated levels of the postprandial bovine BCM7 compared with a healthy control group [19]. The mechanism of action focuses on ss-casein A1 and related forms preferentially that are able to produce a bioactive opioid peptide, ss-casomorphin-7 (ss-CM-7) during digestion. Infants may absorb ss-CM-7 due to an immature gastrointestinal tract. Casomorphin-7 can potentially affect numerous opioid receptors in the nervous, endocrine and immune systems. The peptides interact with opioid and serotonin receptors which are the known modulators of synaptogenesis [20].
The consumption of bovine milk containing BCM7 may induce inflammatory response in intestine by activating Th2 pathway. According to this approach, BCM7 consumption by autistic children induces gastrointestinal problems, such as abnormalities of the bowel mucosa, dysfunctions associated with intestine permeability and changes in the gut microbiota [21]. In another study, A1 milkderived opioid peptides are to be considered as environmental stressors. It was proposed that, they can lead to disorders in the human intestine by causing reduced proteolytic activity to increased permeability of the intestinal barrier [22] Epidemiological studies have shown that β-CM7 intake may be associated with a risk of type 1 diabetes, ischemic heart disease, autism, otoimmune diseases and some physchological health problems. In one study, elevated level of BCM7 in serum of autistic children was reported. Autism is a developmental disorder, which usually manifests itself in the first years of life. Children with autism exhibit abnormal behavior in social interaction and communication problems (Figure 7).
Figure 8:Bovine Casomorphine [22].
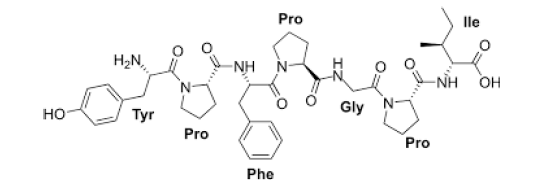
When the opioid peptides pass the intestinal epithelial barrier, which is linked to permeability and the constrained synthesis of dipeptidyl peptidase-4 (DPP4), they in turn can attach to opioid receptors, [23]. Bovine Beta-casomorphins (β-CMs) are opioid peptides encrypted in β-caseins (β-CNs) in an inactive form and released by enzymatic hydrolysis during fermentation and in vivo or in vitro gastrointestinal digestion. These peptides are fragments of the bovine β-CN sequence, 60-70, [24,25].
The exogenous opioid peptides and DPPIV serum activity in infants with apnoea expressed as apparent life-threatening events. In that study, it was suggested the existence of a relation between the occurrence of b-casomorphin-7 in infants’ diets and apnoea in predisposed children [26]. In goats, like in other mammals, four evolutionary conserved casein genes exist. The genes encoding alpha-S1-, beta-, alpha-S2- and kappa-caseins are CSN1S1, CSN2, CSN1S2 and CSN3, respectively [8].
A2 Milk, An Alternative for A1 Milk in Diet
It should be noted that the highest numerical values of the level of balance in terms of organoleptic indicators and energy value were obtained by samples of yoghurts made from a mixture of A1 cow milk, A2 milk and goat’s milk. With respect to food supply, studies have shown that yoghurts made from both cow, goat and their mixed milk acquire the requirements of the standard. The reality about the gastrointestinal effects of A1-type beta-casein protein in the cow milk leading to studies comparing with the progenitor A2 type. Focusing only A1 and A2 makes it seem like there are only two types of cow β-casein protein, but there are actually thirteen peptides. This masks the diversity of caseins and their varying hydrolitic peptides across and within breeds. There are eight β-casein proteins grouped under A2 share the same amino acid at position 67 (proline), whereas the five grouped under A1 have histidine at position 67. This difference in amino acid comes from the digestional differences. Digestive enzymes cut the A1 β-casein types at position 67, producing a seven amino acid-long peptide called β-casomorphin 7 (BCM-7). Because the proline peptide bond with its neighboring amino acids is strong, it resists cleavage so that, A2 β-casein types stay intact at position 67 and no production of BCM-7 occurs [27].
Bovine casein-derived peptides, might be opionic, have been suggested to play a role in sudden infants, so called sudden infant death syndrome (SIDS). In one study, bovine b-casomorphin-7 (bBCM-7) and the activity of dipeptidyl peptidase-IV (DPPIV) in infants with observable life-threatening syndromes. It was found that some infants after an apnoea event contained more b-casomorphin-7 than healthy infants in the same age. In all the children after an apnoea event, DPPIV was depleted. It was proposed that, the casomorphin bBCM-7 in the blood circulation, decreased the activity of peptidase being responsible for opioidinduced respiratory depression. Sudden infant death syndrome (SIDS) is an important cause of deaths in infants [28]. It is supposed that in some cases of SIDS, it is the cow milk that might be the source of the physiologic disorder caused by opioidic peptides. In another study, it was also suspected that beta-casomorphins were directly responsible for that situation [29].
As stated above researches, BCMs are biologically active towards l-opioid (MOP) receptors in central nervous system and gastrointestinal tract. They are agonist with effects similar to that of morphine. and play an important role in responding to stress and pain, in addition to control food intakes [30].
Probiotic Bacteria for the Challenge of Bovine Casomorphin Proteins
It has been known that some probiotic bacteria have protective neurodegenerative effects. Literally, Lactobacillus and Bifidobacter species’ have beneficial effects on human brain health, like delaying Alzheimer occurrence and its progression [31]. Generally, Enzymatic hydrolysis and fermentation, by using probiotic bacteria, are generally emphasized as effective approaches. In the A2 milk, the A2 variant, the peptide bond between proline and isoleucine has more enzymatic resistance than that between histidine and isoleucine in the A1 variant. So, it could be said that, A1 β-casein is more easily hydrolyzed at this residue during digestional process, by gastrointestinal enzymes. So that, as presented in the Figure 8, BCM7 is released, as a consequence, BCM7 is liberated in the digestive tract from β-caseins containing histidine residue at 67 positions, including A1 as well as B β-casein [32].
Figure 9:Bovine Casomorphine [22].
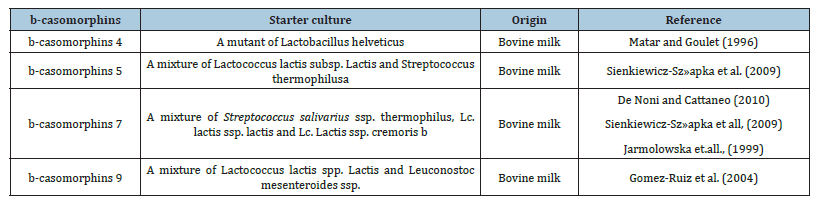
It is postulated in the literature that, the degradative capabilities of 29 Bifidobacterial spp. were analyzed. In those researches, the peptidase enzymatic activity in food-derived opioid peptides were observed and it was stated that some Bifidobacterim longum subsp. in infants have peptidases to degrade casomorphin peptides. According to that study, Bifidobacterial spp’s have potentials to eliminate the casein derived opioid peptides in human intestines. B. longum subsp. Infantis and B. bifidum strains have potential for eliminating milk food-derived opioid peptide. So, further investigations were needed to analyze new degradative enzymes from other probiotic bacterial cells. Because varying degradative capabilities of peptidase enzymes were observed among strains isolated from different stages of life in human (infant, adult, elderly…). As shown in Figure 2, the strains isolated from infants seemed more active, although the difference was not as marked as we having expected in regard to the origin of these B. bifidum strains (Table 1).
Table 1:BCMs relased by peptidase enzymes by different bacteria in the starter cultures.
Conventional approaches, such as enzymatic hydrolysis and fermentation by specific probiotic bacterial spps like Bifidobacteria and Lactobacillus spps’ provide us with a promise as do emerging technologies too. The results of the literatur studies also implied a novel probiotic property of selected Bifidobacterial spp., as an enzyme supplements, to improve the human digestional health problems, which lead to chronic diseases.
Conclusion
There have globally been 20-30-fold increases in the percentages of autistic disorders in human health in the last 40-50 years [35]. The prevalence of autism spectrum disorders is different in worldwide from 1.4/10,000 children in the Arabian Peninsula, 185/10,000 children of Asian population, the highest prevalence has been observed in Sweden in Europe (115/10,000). The lowest prevalence has been in Croatia (2-3/10,000). The occurrence of autism is ranging from three-to-five times higher in males than females [36]. Milk protein derived opioid peptides and DPPIV are potentially factors in occurrences of the pathogenesis of autism. Related studies are still limited and require further research. The adverse effects of BCM-7 on human health still needs further rigorous research. For comprehensive understanding and the development of effective strategies to overcome these adverse effects, including the use of UV-C radiation. Additionally, bioinformatics tools have proven valuable in guiding. Some strategies discussed include genetic selection for ruminants, preliminarily cattle, producing β-casein A2 milk by innovative milk processing methods [37-50]. The aim is to reduce the several types of casomorphin peptides, the mostly found among them is BCM-7 peptide in bovine milk. Naturally, enzymatic hydrolysis and fermentative ways using some probiotic bacterial species, Lactic acid bacteria (Lactobacillus spp.), Bifidobacteria are the ones assessed as effective approaches for their both protective and therapeutic effects.
References
- Faye B, Konuspayeva G (2012) The sustainability challenge to the dairy sector-the growing importance of non-cattle milk production worldwide. Int Dairy J 24(2): 50-56.
- Davies DT, Holt C, Christie WW (1983) The composition of milk Ch 5 in Biochemistry of Lactation TB Mepham, editor, Amsterdam: Elsevier.
- Evershed RP, Payne S, Sherratt AG, Copley MS, Coolidge J, et al. (2008) Earliest date for milk use in the near East and southeastern Europe linked to cattle herding. Nature 455(7212): 528-531.
- Grand Pierre C, Ghisolfi J, Thomsent TH (1988) Biochemical study of goat's milk. Edition Nutr Diet 23: 364-374.
- Cheng Li, Zhiqian Liu, Leah Marett, Jennie Pryce, Simone Rochfort, et al. (2022) Comparison of workflows for milk lipid analysis: Phospholipids. Foods 12(1):163.
- Sarode AR, Sawale PD, Khedkar CD, Kalyankar SD, Pawshe RD, et al. (2016) Casein and caseinate: Methods of manufacture, Ancylopedia of Food and Milk 675-682.
- De Wit JN (1981) Structure and junction al behavior of whey proteins. Netherlands Milk and dairy journal 35.
- Martin P, Szymanowska M, Zwierzchowski L, Leroux C (2002) The impact of genetic polymorphisms on the protein composition of ruminant milks. Reprod Nutr Dev 42(5): 433-59.
- Farrell HMJ, Jimenez Flores R, Brown EM, Buttler JE, Creamer LK, et al. (2004) Nomenclature of the proteins of cow’s milk-Sixth revision. J Dairy Sci 87(6): 1641-1674.
- Danielle G Lemay, David J Lynn, William F Martin, Margaret C Neville, Theresa M Casey, Gonzalo Rincon, et al. (2009) The bovine lactation genome: Insights into the evolution of mammalian milk. Genome Biology 10(4): R43.
- Rijnkels M (2002) Multispecies comparison of the casein gene loci and evolution of casein gene family. Journal of Mammary Gland Biology and Neoplasia 7(3): 327-345.
- Gaunt SN (1980) Genetic variation in the yields and contents of milk constituents. Int Dairy Fed Bull Doc 125: 73.
- Ferretti L, Leone P, Sgaramella V (1990) Long range restriction analysis of the bovine casein genes. Nucleic Acids Research, 18(23): 6829-6833.
- Cieślińska A, Kamiński S, Kostyra E, Sienkiewicz SE (2007) Beta casomorphin 7 in raw and hydrolyzed milk derived from cows of alternative beta-casein genotypes. Milchwissenschaft 62: 125-127.
- Peram MR, Loveday SM, A Ye, SinghIn H (2013) Invitro gastric digestion of heat-induced aggregates of β-lactoglobulin .Journal of Dairy Science 96(1): 63-74.
- Ng Kwai Hang KF, Grosclaude F (2002) Genetic polymorphism of milk proteins. FoxPF, McSweeney PLH (eds). Advanced Dairy Chemistry: Volume 1: Proteins, Parts A &B. Kluwer Academic/Plenum Publishers: New York, pp. 739-816.
- Ewa Fiedorowicz, Maciej Kaczmarski, Anna Cieślińska, Edyta Sienkiewicz S, Beata Jarmołowska, et al. (2014) β casomorphin-7 altersµ-opioid receptor and dipeptidyl peptidase IV genes expression in children with atopic dermatitis. Peptides 62: 144-149.
- Beata Jarmołowska, Marta Bukało, Ewa Fiedorowicz, Anna Cieślińska, Natalia KK, et al. (2019) Role of milk-derived opioid peptides and proline dipeptidyl peptidase-4 in autism spectrum disorders. Nutrients 11(1): 87.
- Whiteley P, Shattock P (2002) Biochemical aspects in autism spectrum disorders: Updating the opioid-excesstheory and presenting new opportunities for biomedical intervention. Expert Opin Ther Targets 6(2): 175-183.
- Oleg Sokolov, Natalya Kost, Olga Andreeva, Ekaterina Korneeva, Viktor Meshavkin, et al. (2014) Autistic children display elevated urine levels of bovine casomorphin-7immunoreactivity. Peptides 56: 68-71.
- Haq MRU, Kapila R, Sharma R, Saliganti V, Kapila S, et al. (2014) Comparative evaluation of cow β-casein variants(A1/A2) consumption on Th2-mediated inflammatory response in mouse gut. Eur J Nutr 53: 1039-1049.
- Keith Bernard Woodford (2021) Casomorphins and gliadorphins have diverse systemic effects spanning gut, brain and internal organs. Int J Environ Res Public Health 18(15): 7911.
- Asledottir T, T Le, Petrat Melin B, TG Devold, LB Larsen, et al. (2017) Identification of bioactive peptides and quantification of β-casomorphin-7 from bovine β-casein A1, A2 and I after ex vivo gastrointestinal digestion. Int Dairy J 71: 98-106.
- De Noni I (2008) Release of β-casomorphins 5 and 7 during simulated gastro-intestinal digestion of bovine β-casein variants and milk-based infant formulas. Food Chem110: 897-903.
- Jolanta Wasilewska, Edyta Sienkiewicz S, Ewa Kuźbida, Beata Jarmołowska, Maciej Kaczmarski, et al. (2011) The exogenous opioid peptides and DPPIV serum activity in infants with apnoea expressed as apparent life threatening events (ALTE). Neuropeptides 45(3): 189-195.
- Vincent D, Elkins A, Condina MR, Ezernieks V, Rochfort S, et al. (2016) Quantitation and identification of intact major milk proteins for high-throughput LC-ESI-Q-TOF MS analyses. PloS one 11(10): e0163471.
- Carpenter RG, Irgens LM, Blair PS, England PD, Fleming P, et al. (2004) Sudden unexplained infant death in 20 regions in Europe: case control study. Lancet 363: 185-191.
- Storm H, Reichelt CL, Rognum TO (1990) Beta-endorphin, human caseomorphin and bovine caseomorphin immunoreactivity in CSF in sudden infant death syndrome and controls. Prog Clin Biol Res 328: 327-330.
- Teschemacher H (2003) Opioid receptor ligands derived from food proteins. Curr Pharm Des 9(16): 1331-1344.
- Sharma A, IJ Martins (2023) The role of microbiota in the pathogenesis of Alzheimer’s disease. Acta Scientific Nutritional Health 127(4): 954-967.
- Jinsmaa Y, Yoshikawa M (1999) Enzymatic release of neocasomorphin and [beta]-casomorphin from bovine [beta]-casein. Peptides 20(8): 957-962.
- Woodford K (2009) Devil in the milk: Illness, Health and Politics of A1 and A2 Chelsea Green Publishing Company, USA.
- Messens W, Estepar Garcia J, Dewettinck K, Huyghebaert A (1999) Proteolysis of high-pressure-treated gouda cheese. Int Dairy J 9(11): 775-782.
- Lefier D, Lamprell H, Mazerolles G (2000) Evolution of Lactococcus strains during ripening in Brie cheese using Fourier transform infrared spectroscopy. Lait 80(2): 247-254.
- Karolina Skonieczna Z, Izabela Gorzkowska, Joanna Pierzak Sominka, Grażyna Adler (2017) The prevalence of autism spectrum disorders in West Pomeranian and Pomeranian regions of Poland. J Appl Res Intellect Disabil 30(2): 283-289.
- Baio J (2010) Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators, Centers for Disease Control and Prevention (CDC). Prevalence of autism spectrum disorder among children aged 8 years-Autism and developmental disabilities monitoring network, United States 2014 (63): 1-21.
- Mc Lachlan CNS, (2001) β-casein A1, ischaemic heart disease mortality, and other illnesses. Med Hypotheses 56(2): 262-272.
- De Noni, Stuknytė M, Cattaneo S (2015) Identification of β-casomorphins 3 to 7 in cheeses and in their invitro gastrointestinal digestates. LWT Food Sci Technol 63(1): 550-555.
- Elliott, Harris DP, Hill JP, Bibby NJ, Wasmuth HE, et al. (1999) Type I (insulin-dependent) diabetes mellitus and cow milk: Casein variant consumption. Diabetologia 42(3): 292-296.
- Jardin G, Airinei B, Marsset (2013) Sequential release of milk protein-derived bioactive peptides in the jejunum in healthy humans. Am J Clin Nutr 97(6): 1314-1323.
- Laugesen MR (2003) ElliottIschaemic heart disease, Type 1 diabetes and cow milk A1 β-casein NZ Med J 116: 1-19.
- Leischner C, Egert S, Burkard M, Venturelli S (2021) Potential protective protein components of cow’s milk against certain tumor entities. Nutrients 13(6): 1974.
- Neha Thakur, Geeta Chauhan, Mishra BP, Mendiratta SK, Pattanaik AK, et al. (2020) Comparative evaluation of feeding effects of A1 and A2 cow milk derived casein hydrolysates in diabetic model of rats. Journal of Functional Foods 75: 104272.
- McMahon DJ, Oommen BS (2008) Supramolecular structure of the casein micelle. J of Dairy Science 91(5): 1709-1721.
- Martin P, Ollivier Bousquet M, Grosclaude F (1999) Genetic polymorphism of caseins: A tool to investigate casein micelle organization. Int Dairy 9(3-6): 163-171.
- Sakurai T, Yamada A, Hashikura N, Odamaki T, Xiao JZ, et al. (2018) Degradation of food-derived opioid peptides by bifidobacteria. Beneficial Microbes 9(4): 675-682.
- Stanislava Yu Petrova, Svetlana V Khlgatian, Olga Yu Emelyanova, Larisa A Pishulina, Valentina, et al. (2022) Structure and biological functions of milk caseins. Russian Open Medical Journal 11(2).
- Trejo Daniel Romero, Sanchez IA, Benny MB, Steider W, Sierra EM, et al. (2024) Anti-cancer potential of casein and its derivatives: Novel strategies for cancer treatment. Med Oncology 41(8): 200.
- Villa C, Costa J, Oliveira MBPP, Mafra I (2018) Bovine milk allergens: A comprehensive review. Compr Rev Food Sci Food Saf 17(1): 137-164.
- Ye A, Liu W, Cui J, Kong X, Roy D, et al. (2019) Coagulation behaviour of milk under gastric digestion: Effect of pasteurizationand ultra-high temperature treatment. Food Chem 286: 216-225.
© 2025 Necibe Canan USTA. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






