- Submissions

Full Text
Novel Techniques in Nutrition and Food Science
The Effect of Lycopene on Reducing the Risk of Prostate Cancer: A Systematic Review and Meta-Analysis
Wanlapa Sakritthichai¹*, Charnsiri Segsarnviriya², Kittibhum Kawinchotpaisan3, Piyameth Dilokthornsakul4 and Thunnawat Wattanaseth1
1Department of Anti-Aging and Regenerative Medicine, College of Integrative Medicine, Dhurakij Pundit University, Thailand
2Department of Otorhinolaryngology, Head and Neck Surgery, Samitivej Thonburi Hospital, Thailand
3Department of Emergency Medicine, Navavej International Hospital, Thailand
4Faculty of Pharmacy, Chiangmai University, Thailand
*Corresponding author:Wanlapa Sakritthichai, College of Integrative Medicine, Dhurakij Pundit University, 110/1-4 Prachachuen Rd, Laksi, Bangkok 10210, Thailand.
Submission: June 4, 2025;Published: June 16, 2025

ISSN:2640-9208Volume8 Issue 3
Abstract
Background: Prostate cancer is a major cause of cancer-related death in men globally. Lycopene, a
carotenoid found in tomatoes and related products, has strong antioxidant properties and may reduce
prostate cancer risk.
Methods: A systematic review and meta-analysis were conducted using PubMed,
Scopus, Cochrane Library and ScienceDirect up to September 2024. Twenty-three observational studies
involving 414,128 participants and 33,552 prostate cancer cases. Pooled odds ratios (ORs) and 95%
confidence intervals (CIs) were calculated using random-and fixed-effects models.
Results: High dietary lycopene intake was significantly associated with reduced prostate cancer risk
(OR=0.91, 95% CI: 0.84-0.99, P=0.03), especially at >10 mg/day. Lycopene intake also reduced advanced
prostate cancer risk (OR=0.88, 95% CI: 0.78-0.99, P=0.04), notably at >15 mg/day. Serum lycopene levels
were associated with prostate cancer risk (OR = 0.83, 95% CI: 0.73-0.96, P = 0.009). Greater risk reduction
was seen in studies with >10 years follow-up (OR=0.92, P = 0.003).
Conclusion: Higher lycopene intake and serum levels are associated with reduced prostate cancer risk.
Notably, lycopene intake also appears to decrease the risk of advanced prostate cancer. These findings
highlight its potential as a preventive dietary factor.
Keywords:Lycopene; Prostate cancer; Meta-analysis; Antioxidant
Abbreviations: CI: Confidence Interval; OR: Odds Ratio; PCA: Prostate Cancer; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; REVMAN: Review Manager; NOS: Newcastle- Ottawa Scale; PCA: Prostate Cancer; NCC: Nested Case-Control; CC: Case-Control; FFQ: Food Frequency Questionnaire; Q: Quantile; FHPC: Family History of Prostate Cancer; BMI: Body Mass Index; NA: Not Applicable; CLUE I: Give us a CLUE to Cancer Study I; CLUE II: Give us a CLUE to Cancer Study II; NSAIDS: Non-Steroidal Anti-Inflammatory Drugs; RCTs: Randomized Controlled Trials
Introduction
Prostate cancer is a significant public health issue and ranks among the most frequently diagnosed malignancies in men globally. It is the second most common cancer and the fifth leading cause of cancer related death among men worldwide, accounting for an estimated 1.4 million new cases and approximately 375,000 deaths annually [1]. While early-stage prostate cancer often has an excellent prognosis, advanced stages are associated with poorer outcomes and significantly diminished quality of life. Current treatment modalities, including androgen deprivation therapy, chemotherapy and radiation, may offer disease control but are frequently accompanied by substantial adverse effects, recurrence, or resistance [2,3]. These limitations underscore the need for preventive strategies that are both effective and minimally invasive. Over the past two decades, the role of dietary factors in modulating cancer risk has gained increasing attention, particularly the consumption of fruits, vegetables and plant-derived compounds known as phytochemicals. Lycopene, a non-provitamin A carotenoid found predominantly in tomatoes and tomato-based products, has emerged as one of the most extensively studied phytochemicals in the context of prostate cancer [4,5]. Unlike beta-carotene, lycopene does not convert into vitamin A but exhibits potent antioxidant properties through its ability to scavenge Reactive Oxygen Species (ROS) [6-8]. These oxidative species contribute to DNA damage, lipid peroxidation and protein dysfunction, processes that are central to carcinogenesis. Furthermore, chronic inflammation, a recognized driver of prostate cancer progression, may be attenuated by lycopene’s anti-inflammatory effects [9]. Biochemically, lycopene exerts multifaceted protective effects through both antioxidant dependent and independent mechanisms. It enhances intracellular gap junction communication, induces cell cycle arrest in the G0/G1 phase, promotes apoptosis and modulates several key molecular pathways, including NF-κB, IGF-1 and androgen receptor signaling. Additionally, lycopene influences gene expression by downregulating oncogenes and upregulating tumor suppressor genes such as p53 and PTEN. Its bioavailability is improved by food processing and the presence of dietary fats, making cooked tomato products a more efficient source of lycopene than raw tomatoes [10].
Although in-vitro and in-vivo studies have consistently demonstrated lycopene’s anticancer potential [11,12], evidence from epidemiological studies remains inconclusive [13]. Some observational studies suggest a significant inverse association between lycopene intake or serum levels and prostate cancer risk, while others report no statistically significant relationship. These discrepancies may stem from methodological differences, including variations in dietary assessment tools, the use of Food Frequency Questionnaires (FFQs), heterogeneous study populations and differences in study design, such as cohort versus case-control or nested case-control studies [14]. Furthermore, variations in follow-up duration and the extent of statistical adjustment for confounding variables may influence reported outcomes [15]. Given these inconsistencies, a comprehensive synthesis of the available evidence is warranted. Systematic reviews and meta-analyses serve as valuable tools for integrating findings across studies, enhancing statistical power and generating more robust conclusions. By pooling data from multiple sources, these methods can clarify the extent and nature of the association between lycopene exposure and prostate cancer risk. This study aims to conduct a systematic review and meta-analysis of observational studies to examine the relationship between lycopene consumption (both dietary and serum levels) and the risk of prostate cancer. Specifically, the analysis will evaluate dose-response relationships, the potential protective effect against advanced prostate cancer. This evidencebased synthesis will contribute to a more refined understanding of lycopene’s role in prostate cancer prevention and may support its inclusion in dietary recommendations for at-risk populations.
Materials and Methods
Protocol and registration
This systematic review and meta-analysis were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [16]. The study protocol was prospectively registered in PROSPERO (International Prospective Register of Systematic Reviews) under the registration number CRD420251053002.
Search strategies
A comprehensive literature search was performed across four electronic databases: PubMed, Scopus, Cochrane Library and ScienceDirect, covering studies published up to 30 September 2024. Search terms included combinations of (1) “Lycopene” or “Tomato” (2) “Prostate cancer” were employed to optimize search precision. Search queries were tailored to the indexing system of each database. In addition, the reference lists of all included studies and relevant systematic reviews were manually screened to identify any eligible trials not captured in the initial search. No language restrictions were applied during the search process. All records were independently screened by two reviewers (W.S. and C.S.) and discrepancies were resolved through discussion or adjudication by a third reviewer.
Eligibility criteria and study selection
The study included observational studies (cohort, nested
case-control and case-control) published in English that met the
following eligibility criteria:
A. Population/participants: Men aged 40 years or older.
B. Intervention: Lycopene with no restrictions on type,
formulation, dose, frequency, or duration of therapy.
C. Comparison/control group: Participants who did not
engage in lycopene. They may have performed usual care.
D. Outcomes: clearly defined diagnosis of prostate cancer.
Data extraction
Data was independently extracted by two reviewers (W.S. and C.S.) using a predesigned standardized data extraction form. Extracted data included: study characteristics (author, year and country), participant demographics (age and sample size), Assessment method (FFQ or serum lycopene) and outcome assessment (diagnosed with confirmed prostate cancer). Discrepancies between reviewers were resolved through discussion and a third reviewer was consulted if consensus could not be reached. When data were missing or unclear, corresponding authors were contacted for clarification. If no response was received, only available data from the publication were used in the analysis.
Quality assessment
The risk of bias for each included observational studies was independently assessed by two reviewers using the Newcastle- Ottawa Scale (NOS) [17]. This tool evaluates three domains: (1) Selection of study groups, (2) Comparability of groups based on design or analysis, (3) Outcome/exposure ascertainment. Each study could score up to 9 points. Studies scoring 7-9 were considered high quality, 5-6 moderate quality and < 5 low quality.
Statistical analysis
All statistical analyses were performed using Review Manager (RevMan) version 5.4. Meta-analyses were conducted based on the degree of heterogeneity among studies. If I²<50%, a fixedeffects model was used; if I²≥50%, a random-effects model (Der Simonian and Laird method) was applied to account for betweenstudy variability. The pooled effect estimate was calculated as the Odds Ratio (OR) with 95% Confidence Interval (CI), comparing the highest vs. lowest lycopene exposure categories. For studies reporting Relative Risk (RR) or Hazard Ratios (HR), these were considered approximately equivalent to OR, based on the rare disease assumption (prostate cancer incidence<10%) [18]. Between-study heterogeneity was quantified using the I² statistic. The degree of heterogeneity was categorized into three levels: low (I²<25%), moderate (I²=25%-75%) and high (I²>75%). Subgroup analyses were conducted according to exposure type (dietary VS serum lycopene), lycopene dose categories (<5mg/day, 5-10mg/ day and>10mg/day), duration of follow-up (<10 years vs. ≥10 years). Potential publication bias was assessed through funnel plot visualization and quantitatively using Egger’s test, with p<0.05 indicating statistically significant bias.
Results
Search results
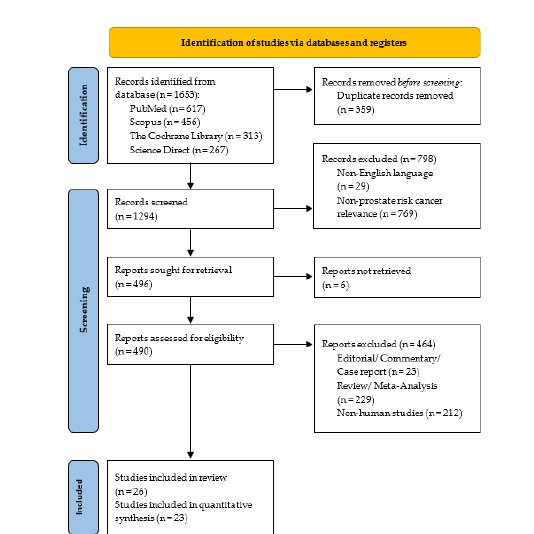
The initial database search retrieved a total of 1,653 records: 617 from PubMed, 456 from Scopus, 313 from the Cochrane Library and 267 from ScienceDirect. After removing 359 duplicates, 1,294 records remained for title and abstract screening. Following this, 769 articles were excluded for not addressing prostate cancer risk and 29 non-English studies were removed. Of the remaining 496 articles, 6 full-text articles were not retrievable. A further 464 studies were excluded for being editorials, reviews, or non-human studies. Ultimately, 26 studies met the eligibility criteria, of which 3 were conducted in overlapping populations. After excluding these, 23 primary studies [19-41] were included in the systematic review and meta-analysis. The study selection process is illustrated in the PRISMA flow diagram (Figure 1).
Figure 1:PRISMA flow diagram of literature search and study selection.
Study characteristics
The 23 included studies comprised 9 cohort studies, 9 nested case-control studies and 5 case-control studies, conducted between 1995 and 2022. The study population comprised 414,128 male participants aged over 40 years, of whom 33,552 were diagnosed with confirmed prostate cancer. Lycopene exposure was assessed either via dietary intake using validated Food Frequency Questionnaires (FFQs) or through serum lycopene levels. Most studies used histologically or clinically confirmed diagnoses of prostate cancer as the outcome of interest. Detailed study characteristics, including location (Specifies the country or region where the study was conducted e.g., USA, Netherlands, Finland or 8 European countries), study design (cohort, nested casecontrol or case-control), follow-up duration, studied population, assessment method and outcome assessment. This table highlights the methodological diversity across studies, which is essential for interpreting pooled results in Table 1. The detailed outcomes of lycopene intake and its associated odds ratio for prostate cancer risk presenting a comparative analysis of various studies evaluating the association between different levels of dietary lycopene intake (measured in mg/day) and odds ratio for overall, advanced and non-advanced prostate cancer. For each study, showing lycopene doses is categorized into quantiles. The reference category (Q1) is used as the baseline. The odds ratios and their corresponding 95% CI for higher quantiles (Q2-Q5) are presented relative to this baseline in Table 2. The detailed outcomes of serum lycopene and its associated odds ratios for prostate cancer risk presenting a comparative analysis of various studies evaluating the association between different levels of serum lycopene (measured in μg/dl) and odds ratio for overall, advanced and non-advanced prostate cancer. For each study, serum lycopene levels are categorized into quantiles. The reference group (Q1) represents the lowest exposure and serves as the baseline. The odds ratios and their corresponding 95% CI for higher quantiles (Q2-Q5) are presented relative to this baseline in Table 3.
Table 1:Characteristics of included studies.
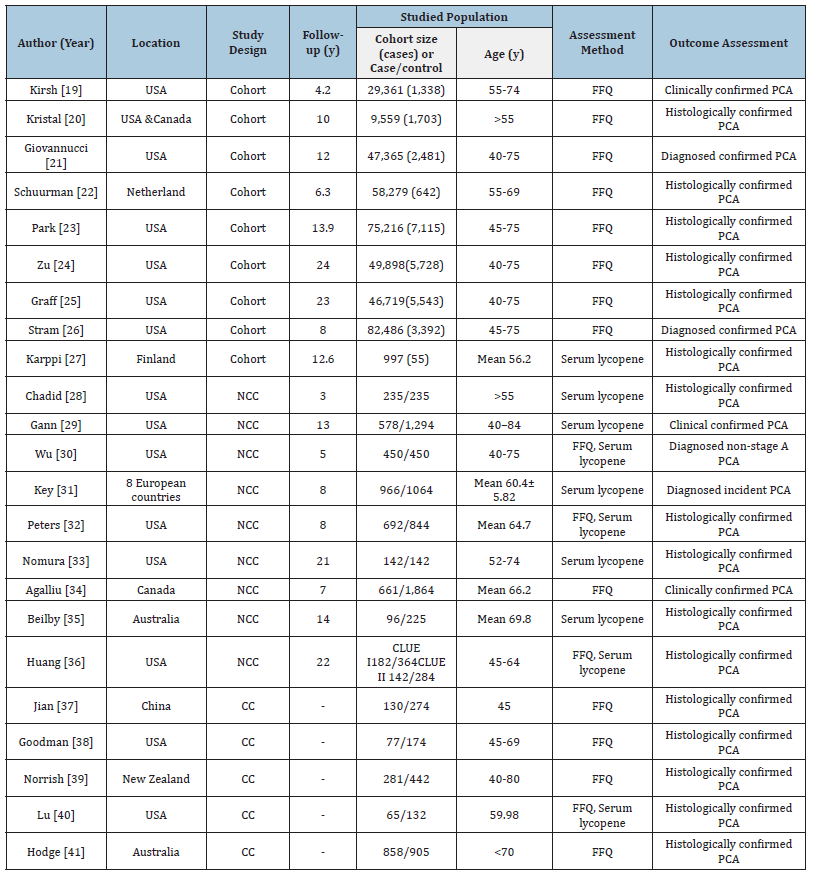
Table Abbreviations: NCC: Nested Case-Control, CC: Case-Control, FFQ: Food Frequency Questionnaire, Pca, prostate cancer, CLUE I, give us a CLUE to Cancer Study I, CLUE II, Give us a CLUE to Cancer Study II.
Table 2:Detailed Outcomes on Lycopene Intake and Odds Ratio of Prostate Cancer.
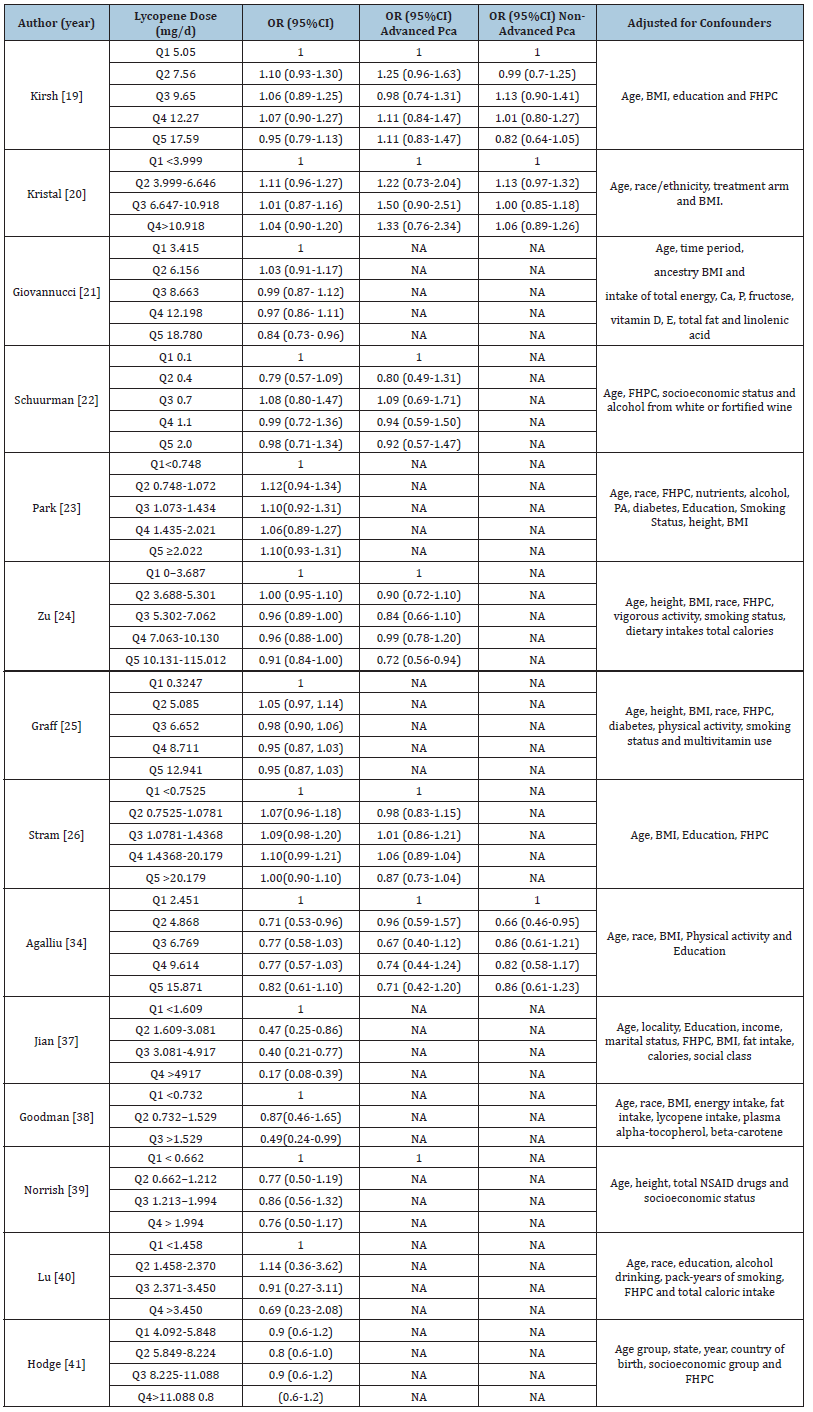
Table Abbreviations: Q: Quantile, OR: Odds Ratio, NA: Not Applicable, CI: Confident Interval, PCA: prostate cancer, BMI: Body Mass Index, FHPC: Family History of Prostate Cancer, NASIDS: Non-Steroidal Anti-Inflammatory Drugs
Table 3:Detailed Outcomes on Serum Lycopene and Odds Ratio of Prostate Cancer.
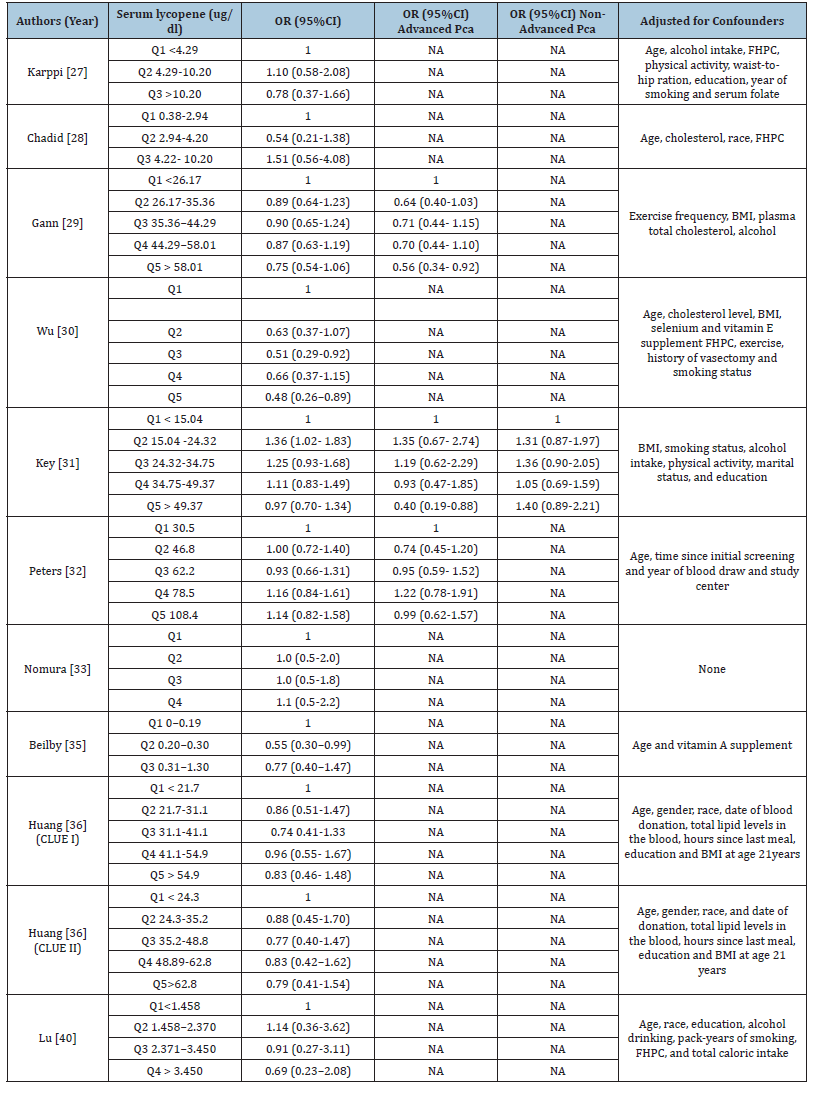
Table Abbreviations:Q: Quantile, OR: Odds Ratio, NA: Not Applicable, CI: Confident Interval, PCA: Prostate Cancer, BMI: Body Mass Index, FHPC: Family History of Prostate Cancer, NASIDS: Non-Steroidal Anti-Inflammatory Drugs.
Quality assessment of included studies
The quality of 23 studies included comprising 9 cohort, 9
nested case-control and 5 case-control studies-was evaluated
using the Newcastle-Ottawa Scale (NOS), a standardized tool for
assessing non-randomized studies. Most studies demonstrated
good methodological quality.
Cohort studies: All 9 cohort studies included, 7 achieved a
full NOS score of 9, indicating high quality. Kirsh [19] scored 7 due
to a short follow-up period and unclear reporting. Cohort designs
offer strong causal inference by establishing temporal relationships
between exposure and outcome. These studies demonstrated
rigorous population selection and control for confounders,
supporting their reliability for quantitative synthesis.
Nested case-control studies: All 9 nested case-control studies
scored between 7 and 9 on the NOS. Most scored 8 or 9, indicating
high quality. The studies that scored 8 points were those by Wu
[30], Agalliu [34] and Chadid [28], primarily due to missing data on
non-response rates or loss to follow-up. Nomura [33] and Key [31]
scored 7 due to insufficient confounder adjustment and unclear
non-response reporting. Overall, these studies showed robust
design and valid exposure assessment, justifying inclusion in metaanalysis.
Case-control studies: The 5 case-control studies scored
between 7 and 9, reflecting generally good quality. Goodman and Lu
[40] scored 7 due to concerns about sample representativeness and
response rate imbalance. Nonetheless, all case-control studies met
minimum quality standards for inclusion in the analysis. Overall
Assessment: most studies were of good quality (NOS≥7). Cohort
and nested case-control studies had slightly higher average scores
than case-control studies, consistent with their stronger design
features in reducing bias. The quality of included studies supports
the credibility of the meta-analysis findings in Tables 4 & 5.
Table 4:Quality assessment of studies included in meta-analyses, using the Newcastle Ottawa scale for cohort studies
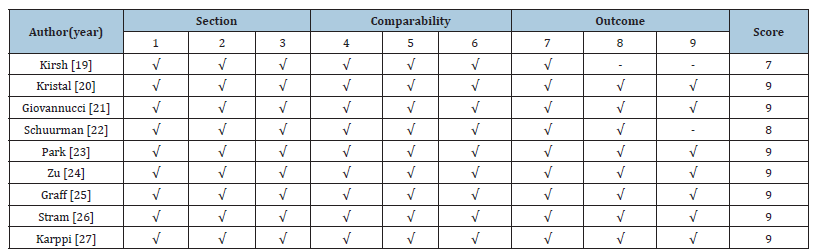
Remarks:
1.The lycopene-consuming group should be representative of the general population.
2.The comparison group (non-lycopene consumers) should be selected from the same source population. 3: Lycopene
intake should be measured using reliable methods, such as a validated food frequency questionnaire (FFQ) or serum
lycopene levels.
3.Participants must be confirmed to be free of prostate cancer at the start of the study.
4.Age, as a key confounding factor, must be adequately controlled.
5.Other potential confounders such as body mass index (BMI), smoking status and family history should also be
controlled.
6.The diagnosis of prostate cancer must be reliable, preferably confirmed through biopsy or cancer registry data.
7.A minimum follow-up duration of five years is recommended to allow sufficient time for disease development.
8.Follow-up data should be adequate, with minimal loss to follow-up.
Table 5:Quality assessment of studies included in meta-analyses, using the Newcastle Ottawa scale for nested casecontrol and case-control studies.
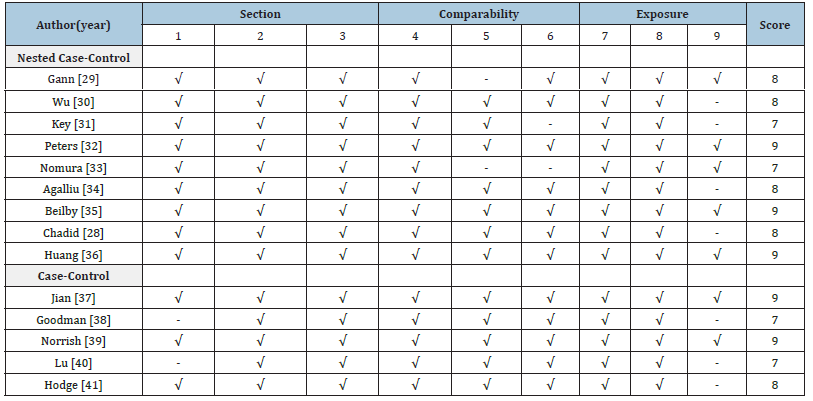
Remarks:
1.The lycopene-consuming group should be representative of the general population.
2The case group should adequately represent the at-risk population.
3.The control group should be selected from the same population and must be free of prostate cancer.
4.Controls must be confirmed to be free of prostate cancer at the time of selection.
5.Age, as a key confounding factor, must be appropriately controlled.
6.Other potential confounders, such as body mass index (BMI), smoking and family history, should also be adjusted for.
7.Lycopene intake should be assessed using reliable methods, such as a validated food frequency questionnaire (FFQ)
or serum lycopene concentration.
8.The same measurement methods should be applied to both cases and controls.
9.The non-response rates between cases and controls should be comparable.
Meta-analysis
A total of twenty-three observational studies comprising 414,128 participants, were included in the meta-analysis.
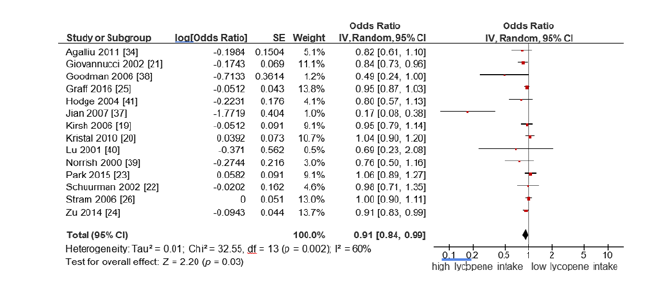
Lycopene intake and prostate cancer risk: Fourteen studies evaluated the association between high vs. low lycopene intake and prostate cancer risk. Using a random-effects model due to moderate heterogeneity (I²=60%), the pooled OR was 0.91 (95%CI (0.84- 0.99); p=0.03). This indicates a statistically significant inverse association between high lycopene intake and prostate cancer risk (Figure 2).
Figure 2:Forest plot for the association of the highest vs the lowest lycopene intake on prostate cancer risk.
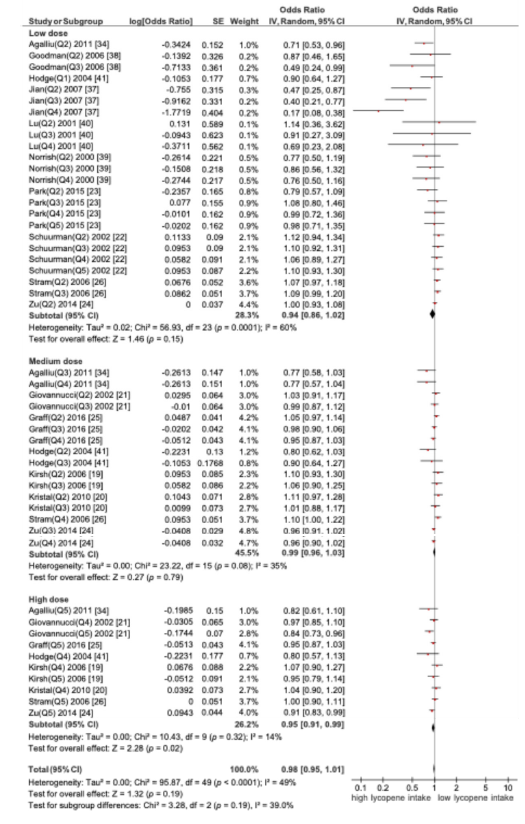
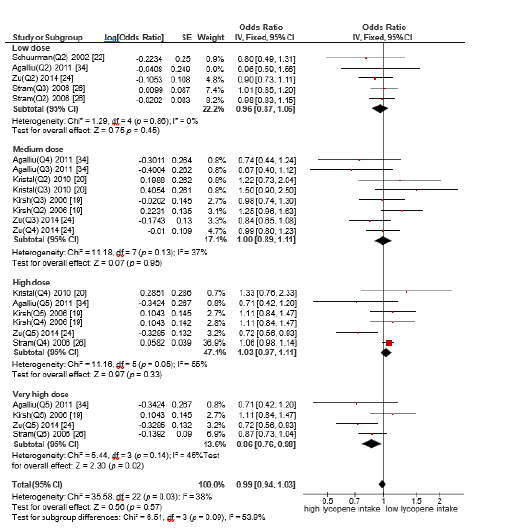
A. Subgroup analysis based on lycopene dose and prostate cancer
A subgroup meta-analysis was conducted to assess the association between dietary lycopene intake and the risk of prostate cancer, stratified by three intake levels: low dose (<5mg/ day), medium dose (5-10mg/day) and high (>10mg/day). Only the high-dose group (>10mg/day) showed a statistically significant reduction in the risk of prostate cancer. OR=0.95 (95% CI (0.91- 0.99); p=0.02), indicating a significant protective effect (Figure 3).
Figure 3:Forest plot of subgroup meta-analysis by lycopene dose and prostate cancer risk.
B. Subgroup analysis based on advanced prostate cancer
A subset of studies focused on advanced prostate cancer
outcomes. Using a fixed-effects model (I²=34%), the pooled OR
was 0.88 (95% CI: 0.78-0.99, p=0.04), indicating that high dietary
lycopene intake was significantly associated with reduced risk of
advanced prostate cancer (Figure 4).
Figure 4:Forest plot for the association of the highest vs the lowest lycopene intake on advanced prostate cancer risk.
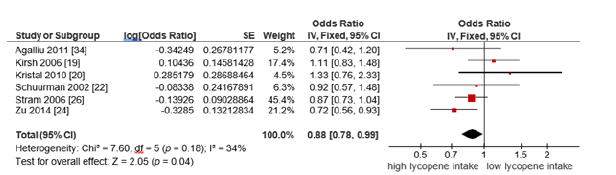
C. Subgroup analysis based on lycopene dose and advanced
prostate cancer
A subgroup meta-analysis was conducted to assess the
association between dietary lycopene intake and the risk of
advanced prostate cancer, stratified by four intake levels: low dose
(<5mg/day), medium dose (5-10 mg/day), high (10-15mg/day)
and very high dose (>15mg/day). Only the very high dose group
(>15 mg/day) demonstrated a statistically significant inverse
association with advanced prostate cancer risk. Odds ratio (OR)
for this group was 0.86 (95% CI: 0.76-0.98, p=0.02), indicating a
significant protective effect. In contrast, no statistically significant
associations were observed for the lower intake categories (Figure
5).
Figure 5:Forest plot for the association of the highest vs the lowest lycopene intake on advanced prostate cancer risk.
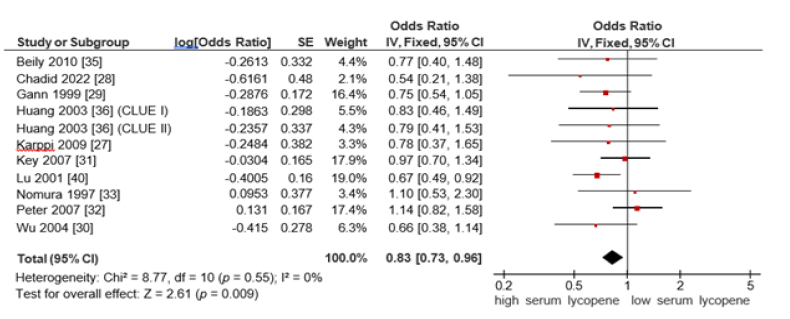
Seum lycopene and prostate cancer risk: Ten studies examined the association between serum lycopene levels and prostate cancer risk. A fixed-effects model was used due to the absence of heterogeneity (I²=0%), indicating a high degree of consistency among the included studies. The pooled OR was 0.83 (95% CI:0.73-0.96, p=0.009), suggesting a statistically significant inverse relationship between high serum lycopene levels and prostate cancer risk (Figure 6).
Figure 6:Forest plot for the association of the highest vs the lowest serum lycopene on prostate cancer risk.
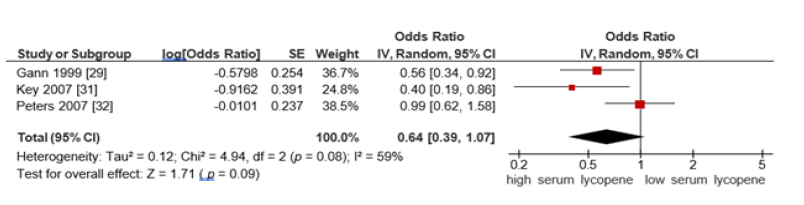
A. Subgroup analysis based on advanced prostate cancer
For advanced prostate cancer, the pooled OR from serum
lycopene studies was 0.64 (95% CI: 0.39-1.07, p =0.09), using a
random-effects model (I²=59%). This did not reach statistical
significance but suggested a protective trend (Figure 7).
Figure 7:Forest plot for association of highest vs lowest serum lycopene on advanced prostate cancer risk.
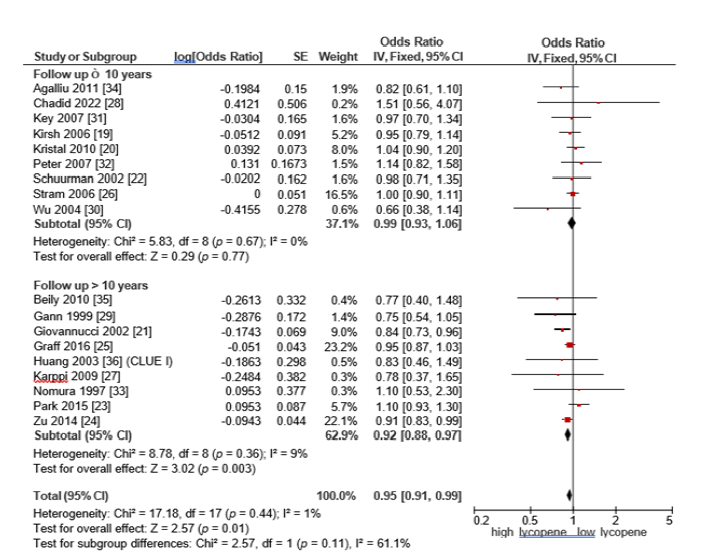
Follow-up duration and prostate cancer risk: Subgroup analyses based on study follow-up duration revealed that subgroup with follow-up duration of 10 years or less, no statistically significant association was observed between lycopene intake and prostate cancer risk. The pooled OR was 0.99 (95% CI: 0.93-1.06, p=0.77), with no observed heterogeneity (I²=0%), indicating a high degree of consistency among the included studies. Conversely, in the subgroup of studies with follow-up periods greater than 10 years, lycopene consumption was significantly associated with a reduced risk of prostate cancer. The pooled OR was 0.92 (95% CI: 0.88-0.97, p=0.003) and the heterogeneity was low (I²=9%), reflecting substantial consistency across studies in this group. These findings suggest that the protective effect of lycopene may become more evident with longer-term exposure, emphasizing the potential importance of sustained dietary lycopene intake for prostate cancer prevention (Figure 8).
Figure 8:Forest plot of subgroup analysis stratified by follow-up duration (≤10 years vs. >10 years) for the association between lycopene intake and prostate cancer risk.
Publication bias
Publication bias was assessed using funnel plots for both dietary and serum lycopene analyses, which showed approximate symmetry, suggesting no major publication bias. The results of Egger’s tests supported these findings across all subgroups: lycopene intake and prostate cancer (Z=0.08, p=0.62), serum lycopene and prostate cancer (Z=-0.01, p=0.94), lycopene intake and advanced prostate cancer (Z=-0.24, p =0.47) and serum lycopene and advanced prostate cancer (Z=0.84, p=0.52). All p-values were greater than 0.05, indicating no statistically significant evidence of publication bias in any subgroup.
Discussion
Prostate cancer is a common and deadly malignancy among men. While early detection offers a high survival rate, advanced disease has a poor prognosis, highlighting the need for effective prevention. Current treatments improve outcomes but face challenges like side effects and recurrence [42]. As a result, attention has shifted toward preventive strategies, particularly dietary approaches. Lycopene, a carotenoid predominantly found in tomatoes, has demonstrated potential anticancer effects, including antioxidant activity [43]. modulation of gene expression and induction of apoptosis in prostate cancer cells [44]. The anti-aging gene Sirtuin 1 has been shown to be activated by lycopene. Sirtuin 1 has been linked with the development of prostate cancer. The role of lycopene levels may be linked to Sirtuin 1 levels to reduce the risk of prostate cancer [45-47]. Observational studies, including cohort, case-control and nested case-control designs, have produced mixed results regarding the association between lycopene and prostate cancer risk. These inconsistencies are attributable to variability in study designs, methods for measuring lycopene intake or blood levels, duration of follow-up and population characteristics. Consequently, this systematic review and meta-analysis aimed to synthesize the existing evidence and clarify the potential protective effect of lycopene against prostate cancer. A total of 23 eligible studies were included, encompassing diverse geographical settings and study populations. Most studies originated from high-income countries and focused on middle-aged to elderly men of predominantly Caucasian ethnicity. Notably, none of the included studies utilized mortality (i.e., prostate cancer-specific deaths) as an outcome, focusing instead on incidence as the primary endpoint. The quality of studies was assessed using the NOS and most studies scored between 7 and 9 points, indicating generally high methodological quality. Cohort studies tended to receive higher scores, reflecting their advantage in establishing temporal relationships and minimizing recall bias. The overall pooled analysis revealed a statistically significant inverse association between high lycopene intake and prostate cancer risk, (OR=0.91, 95% CI: 0.84-0.99, P=0.03). This finding supports the hypothesis that lycopene may exert a protective effect against prostate carcinogenesis [43,44] and aligns with previous research, the meta-analysis by Chen [48], which included 26 studies, reported an inverse association between lycopene consumption and prostate cancer risk. Similarly, Rowles [49] found a significant risk reduction associated with lycopene intake, particularly among individuals with higher levels of consumption. Subgroup analyses provided additional insight into potential dose-response effects. Specifically, only individuals consuming lycopene at levels exceeding 10mg/day showed a significant reduction in risk (OR=0.95, 95% CI: 0.91-0.99, P=0.02) and aligns with previous findings by Zu [24], who reported that each 2mg/day increase in lycopene intake was associated with a 3-5% reduction in prostate cancer risk, whereas lower levels of intake (<10mg/day) did not yield statistically significant results.
The subgroup meta-analysis for advanced prostate cancer revealed a statistically significant inverse association between overall lycopene consumption and disease risk (OR=0.88, 95% CI: 0.78-0.99, P=0.04). This finding highlights the potential of lycopene in delaying or preventing the progression of more aggressive forms of prostate cancer [50]. When stratified by cancer severity, lycopene intake >15mg/day was significantly associated with a decreased risk of advanced prostate cancer (OR=0.86, 95% CI: 0.76-0.98, P=0.02). This supports a biologically plausible dose-response relationship and aligns with previous findings by Zu [24], who reported that each 2mg/day increase in lycopene intake was associated with a 3-5% reduction in prostate cancer risk. However, heterogeneity across studies was moderate (I²=60%), reflecting differences in study methodology, participant characteristics and lycopene assessment methods. In addition to dietary intake, the analysis examined serum lycopene levels in relation to prostate cancer risk. A separate pooled analysis of 10 studies showed that individuals with the highest serum lycopene concentrations had a significantly lower risk of prostate cancer (OR=0.83, 95% CI: 0.73-0.96, P=0.009). The results of this study are consistent with the meta-analysis conducted by Chen [48]. Importantly, heterogeneity among these studies was minimal (I²=0%), increasing confidence in the reliability of this association. Since serum lycopene reflects both dietary intake and bioavailability, this biomarker may serve as a more accurate indicator of lycopene exposure than dietary assessment alone. Although the association between serum lycopene and advanced prostate cancer did not reach statistical significance (OR=0.64, 95% CI: 0.39-1.07, P=0.09), the effect estimate was suggestive of a protective trend. The lack of significance may be attributable to limited sample sizes, inconsistencies in defining advanced disease and variability in serum measurement techniques. Nevertheless, these findings warrant further exploration. Follow-up duration was also a significant modifier of observed associations. Studies with longer follow-up periods (>10 years) demonstrated a clear inverse relationship between lycopene intake and prostate cancer risk (OR=0.92, 95% CI: 0.88-0.97, P=0.003), whereas no association was found in studies with follow-up ≤10 years. This suggests that lycopene’s protective effect may require prolonged exposure and accumulation in tissue before manifesting a measurable benefit [51] and aligns with previous research, the meta-analysis by Rowles [49] This study has several notable strengths. First, the use of systematic review and meta-analytic methodologies reduces selection bias and increases statistical power. Second, subgroup analyses enabled exploration of key moderators such as lycopene dosage, cancer stage and follow-up duration. Third, inclusion of both dietary and biomarker data provides a more comprehensive understanding of lycopene exposure. Finally, the robustness of the findings was reinforced by consistency across studies and minimal evidence of publication bias.
However, limitations must be acknowledged. Most studies assessed lycopene intake using FFQs, which are prone to recall and reporting biases, especially in older populations. Additionally, the observational nature of the included studies precludes definitive causal inference. Although RCTs of lycopene supplementation exist, they remain limited in number, sample size and short follow-up duration, emphasizing the need for further high-quality interventional research. It is recommended that future studies should include participants from developing countries and varied genetic and dietary backgrounds to enhance generalizability. Longterm prospective studies with extended follow-up are needed to capture cumulative effects, with careful adjustment for confounders such as diet, medication use and screening practices. Although observational data offers useful insights, high-quality Randomized Controlled Trials (RCTs) are essential to establish causality. These trials should focus on high-risk groups such as men with elevated PSA or a family history of prostate cancer. Combining FFQ-based dietary assessments with plasma or serum lycopene levels will reduce recall bias and improve exposure accuracy. Additionally, future research should emphasize dose–response analyses to identify intake thresholds necessary for protective effects, guiding both clinical and public health recommendations.
Conclusion
This systematic review and meta-analysis offer comprehensive evidence supporting a protective association between high lycopene intake and a reduced risk of prostate cancer. Protective effects were more pronounced among individuals with higher intake levels and in studies with extended follow-up durations, suggesting a dose and time dependent relationship. Additionally, serum lycopene levels showed an inverse correlation with prostate cancer risk, reinforcing the potential biological relevance of lycopene in inhibiting prostate carcinogenesis. These findings highlight lycopene as a promising, non-pharmacologic, diet-based preventive approach and provide a strong scientific basis for future interventional studies and public health guidelines.
Acknowledgment
The authors gratefully acknowledge the support and assistance provided by Academic Program Officer, Master of Science in Anti- Aging and Regenerative Medicine, during the entire process of this study.
Conflict of Interest
The authors declare no conflicts of interest.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, et al. (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3): 209-249.
- Edwards BK, Noone AM, Mariotto AB, Simard EP, Boscoe FP, et al. (2014) Annual report to the nation on the status of cancer, 1975-2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer 120(9): 1290-314.
- Fizazi K, Faivre L, Lesaunier F, Delva R, Gravis G, et al. (2015) Androgen deprivation therapy plus docetaxel and estramustine versus androgen deprivation therapy alone for high-risk localised prostate cancer (GETUG 12): A phase 3 randomised controlled trial. Lancet Oncol 16(7): 787-794.
- Chan JM, Holick CN, Leitzmann MF, Rimm EB, Willett WC, et al. (2006) Diet after diagnosis and the risk of prostate cancer progression, recurrence and death (United States). Cancer Causes Control 17(2): 199-208.
- Story EN, Kopec RE, Schwartz SJ, Harris GK (2010) An update on the health effects of tomato lycopene. Annu Rev Food Sci Technol 1: 189-210.
- Shi J, Le Maguer M (2000) Lycopene in tomatoes: Chemical and physical properties affected by food processing. Crit Rev Food Sci Nutr 40(1): 1-42.
- Stahl W, Sies H (1996) Lycopene: A biologically important carotenoid for humans? Arch Biochem Biophys 336(1): 1-9.
- Clinton SK (1998) Lycopene: Chemistry, biology and implications for human health and disease. Nutr Rev 56(2): 35-51.
- Rao AV, Agarwal S (1999) Role of lycopene as antioxidant carotenoid in the prevention of chronic diseases: A review. Nutr Res 19(2): 305-323.
- Mirahmadi M, Azimi Hashemi S, Saburi E, Kamali H, Pishbin M, et al. (2020) Potential inhibitory effect of lycopene on prostate cancer. Biomed Pharmacother 129: 110459.
- Rao AV, Rao LG (2007) Lycopene and human health. Curr Top Nutraceutical Res 5(4): 137-144.
- Kavanaugh CJ, Trumbo PR, Ellwood KC (2007) The U.S. food and drug administration's evidence-based review for qualified health claims: Tomatoes, lycopene and cancer. J Natl Cancer Inst 99(14): 1074-1085.
- Rowles JL, Erdman JW (2012) Circulating lycopene and prostate cancer risk: A meta-analysis of epidemiologic studies. Prostate Cancer 2012: 1-8.
- Key TJ, Appleby PN, Allen NE (2004) Nutrition and prostate cancer: A review of the evidence. Br J Nutr 91(6): 1111-1120.
- Etminan M, Takkouche B, Caamaño IF (2004) The role of tomato products and lycopene in the prevention of prostate cancer: A meta-analysis of observational studies. Cancer Epidemiol Biomarkers Prev 13(3): 340-345.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, et al. (2021) The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Bmj 372(71).
- Wells GA, Shea B, O'Connell D, Peterson J, Welch V, et al. (2011) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute.
- Greenland S (1987) Interpretation and choice of effect measures in epidemiologic analyses. Am J Epidemiol 125(5): 761-768.
- Kirsh VA, Mayne ST, Peters U, Chatterjee N, Leitzmann MF, et al. (2006) A prospective study of lycopene and tomato product intake and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 15(1): 92-98.
- Kristal AR, Arnold KB, Neuhouser ML, Goodman P, Platz EA, et al. (2010) Diet, supplement use and prostate cancer risk: Results from the prostate cancer prevention trial. Am J Epidemiol 172(5): 566-577.
- Giovannucci E, Rimm EB, Liu Y, Stampfer MJ, Willett WC, et al. (2002) A prospective study of tomato products, lycopene and prostate cancer risk. J Natl Cancer Inst 94(5): 391-398.
- Schuurman AG, Goldbohm RA, Brants HA, van den Brandt PA (2002) A prospective cohort study on intake of retinol, vitamins C and E and carotenoids and prostate cancer risk (Netherlands). Cancer Causes Control 13(6): 573-582.
- Park SY, Haiman CA, Cheng I, Park SL, Wilkens LR, et al. (2015) Racial/ethnic differences in lifestyle-related factors and prostate cancer risk: The multiethnic cohort study. Cancer Causes Control 26(10): 1507-1515.
- Zu K, Mucci L, Rosner BA, Clinton SK, Loda M, et al. (2014) Dietary lycopene, angiogenesis and prostate cancer: A prospective study in the prostate-specific antigen era. J Natl Cancer Inst 106(2): djt430.
- Graff RE, Pettersson A, Lis RT, Ahearn TU, Markt SC, et al. (2016) Dietary lycopene intake and risk of prostate cancer defined by ERG protein expression. Am J Clin Nutr 103(3): 851-860.
- Stram DO, Hankin JH, Wilkens LR, Park S, Henderson BE, et al. (2006) Prostate cancer incidence and intake of fruits, vegetables and related micronutrients: The multiethnic cohort study. Cancer Causes Control 17(9): 1193-1207.
- Karppi J, Kurl S, Nurmi T, Rissanen TH, Pukkala E, et al. (2009) Serum lycopene and the risk of cancer: The Kuopio Ischaemic Heart Disease Risk Factor (KIHD) study. Ann Epidemiol 19(7): 512-518.
- Chadid S, Song X, Schenk JM, Gurel B, Lucia MS, et al. (2022) Association of serum carotenoids and retinoids with intraprostatic inflammation in men without prostate cancer or clinical indication for biopsy in the placebo arm of the prostate cancer prevention trial. Nutr Cancer 74(1): 141-148.
- Gann PH, Ma J, Giovannucci E, Willett W, Sacks FM, et al. (1999) Lower prostate cancer risk in men with elevated plasma lycopene levels: Results of a prospective analysis. Cancer Res 59(6): 1225-1230.
- Wu K, Erdman JW, Schwartz SJ, Platz EA, Leitzmann M, et al. (2004) Plasma and dietary carotenoids and the risk of prostate cancer: A nested case-control study. Cancer Epidemiol Biomarkers Prev 13(2): 260-269.
- Key TJ, Appleby PN, Allen NE, Travis RC, Roddam AW, et al. (2007) Plasma carotenoids, retinol and tocopherols and the risk of prostate cancer in the European prospective investigation into cancer and nutrition study. Am J Clin Nutr 86(3): 672-681.
- Peters U, Leitzmann MF, Chatterjee N, Wang Y, Albanes D, et al. (2007) Serum lycopene, other carotenoids and prostate cancer risk: A nested case-control study in the prostate, lung, colorectal and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev 16(5): 962-968.
- Nomura AM, Stemmermann GN, Lee J, Craft NE (1997) Serum micronutrients and prostate cancer in Japanese Americans in Hawaii. Cancer Epidemiol Biomarkers Prev 6(7): 487-491.
- Agalliu I, Kirsh VA, Kreiger N, Soskolne CL, Rohan TE, et al. (2011) Oxidative balance score and risk of prostate cancer: Results from a case-cohort study. Cancer Epidemiol 35(4) :353-361.
- Beilby J, Ambrosini GL, Rossi E, de Klerk NH, Musk AW, et al. (2010) Serum levels of folate, lycopene, β-carotene, retinol and vitamin E and prostate cancer risk. Eur J Clin Nutr 64(10): 1235-1238.
- Huang HY, Alberg AJ, Norkus EP, Hoffman SC, Comstock GW, et al. (2003) Prospective study of antioxidant micronutrients in the blood and the risk of developing prostate cancer. Am J Epidemiol 157(4): 335-344.
- Jian L, Lee AH, Binns CW (2007) Tea and lycopene protect against prostate cancer. Asia Pac J Clin Nutr 16(Suppl 1): 453-457.
- Goodman M, Bostick RM, Ward KC, Terry PD, van Gils CH, et al. (2006) Lycopene intake and prostate cancer risk: Effect modification by plasma antioxidants and the XRCC1 genotype. Nutr Cancer 55(1): 13-20.
- Norrish AE, Jackson RT, Sharpe SJ, Skeaff CM (2000) Prostate cancer and dietary carotenoids. Am J Epidemiol 151(2): 119-123.
- Lu QY, Hung JC, Heber D, Go VL, Reuter VE, et al. (2001) Inverse associations between plasma lycopene and other carotenoids and prostate cancer. Cancer Epidemiol Biomarkers Prev 10(7): 749-756.
- Hodge AM, English DR, McCredie MR, Severi G, Boyle P, et al. (2004) Foods, nutrients and prostate cancer. Cancer Causes Control 15(1): 11-20.
- Mohler JL, Antonarakis ES, Armstrong AJ (2019) Prostate cancer, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 17(5): 479-505.
- Rao AV, Agarwal S (2000) Role of antioxidant lycopene in cancer and heart disease. J Am Coll Nutr 19(5): 563-569.
- Story EN, Kopec RE, Schwartz SJ, Harris GK (2010) An update on the health effects of tomato lycopene. Annu Rev Food Sci Technol 1: 189-210.
- Martins IJ (2016) Anti-aging genes improve appetite regulation and reverse cell senescence and apoptosis in global populations. Adv Aging Res 5(1): 9-26.
- Martins IJ (2018) Sirtuin 1, a diagnostic protein marker and its relevance to chronic disease and therapeutic drug interventions. EC Pharmacol Toxicol 6(3): 125-131.
- Jung Hynes B, Nihal M, Zhong W, Ahmad N (2009) Role of sirtuin histone deacetylase SIRT1 in prostate cancer: A target for prostate cancer management via its inhibition? J Biol Chem 284(6): 3823-3832.
- Chen P, Zhang W, Wang X, Zhao K, Negi DS, et al. (2015) Lycopene and risk of prostate cancer: A systematic review and meta-analysis. Medicine (Baltimore) 94(33): e1260.
- Rowles JL, Ranard KM, Smith JW, An R, Erdman JW, et al. (2017) Increased dietary and circulating lycopene are associated with reduced prostate cancer risk: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis 20(4): 361-377.
- Kucuk O, Sarkar FH, Djuric Z (2002) Effects of lycopene supplementation in patients with localized prostate cancer. Exp Biol Med 227(10): 881-885.
- Agarwal S, Rao AV (2000) Tomato lycopene and its role in human health and chronic diseases. CMAJ 163(6): 739-744.
© 2025 Wanlapa Sakritthichai. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






