- Submissions

Full Text
Novel Research in Sciences
The Effectiveness of Platelet- Rich Plasma Therapy in Treating Periorbital Wrinkles: A Systematic Review and Meta-Analysis
Sirilak Sutthinont¹*, Charnsiri Segsarnviriya² and Mart Maiprasert¹
1Department of Anti-Aging and Regenerative Medicine, Dhurakij Pundit University, Thailand
2Department of Otorhinolaryngology, Head and Neck Surgery, Thailand
*Corresponding author:Sirilak Sutthinont, College of Integrative Medicine, Dhurakij Pundit University, 110/1-4 Prachachuen Rd, Laksi, Bangkok 10210, Thailand
Submission: May 28, 2025;Published: June 06, 2025
.jpg)
Volume16 Issue 4June 06, 2025
Abstract
This study aimed to evaluate the effectiveness of Platelet-Rich Plasma (PRP) therapy in reducing periorbital wrinkles through a systematic review and meta-analysis. A comprehensive search of PubMed, Scopus, and The Cochrane Library was conducted until August 2024. The Randomized Controlled Trials (RCTs) that assessed the effects of PRP therapy on periorbital wrinkles were included. Three studies (a total of 58 participants) were included in the meta-analysis. The meta-analysis revealed no statistically significant difference between the PRP treatment group and the control group in terms of the Global Aesthetic Improvement Scale (GAIS), with a risk difference of 0.13 (95% CI: -0.27 to 0.53, p=0.52), indicating that PRP did not make a clear difference in improving patient satisfaction compared to the untreated group. However, sensitivity analysis demonstrated that after excluding the study by Heba M. Diab, the overall result showed a statistically significant difference, with a risk difference of 0.29 (95% CI: 0.02 to 0.57, p=0.037), suggesting a potential beneficial effect of PRP treatment in the absence of this study. Although the overall meta-analysis did not demonstrate a statistically significant improvement in patient satisfaction with PRP treatment compared to control, sensitivity analysis revealed a potential benefit when excluding one outlier study. These findings suggest that PRP may be effective for skin rejuvenation and periorbital wrinkle reduction in selected populations. Further well-designed studies with larger sample sizes, standardized assessment tools, and longer follow-up periods are warranted to confirm its efficacy and safety.
Keywords: Platelet-rich plasma; Periorbital wrinkle reduction; Skin rejuvenation; Crow’s feet; PRP treatment
Abbreviations: PRP: Platelet-Rich Plasma; GAIS: Global Aesthetic Improvement Scale; WSRS: Wrinkle Severity Rating Scale; MFWS: Modified Fitzpatrick Wrinkle Scale; VISIA: VISIA Complexion Analysis System; PPP: Platelet-Poor Plasma; TCA: Tri chloroacetic Acid; RCT: Randomized Controlled Trial; RoB-2: Revised Cochrane Risk of Bias Tool 2; CI: Confidence Interval; RD: Risk Difference; UV: Ultraviolet Radiation
Introduction
Aging of the skin is a natural process that is influenced by both intrinsic [1] and extrinsic [2] factors. Intrinsic aging, [3-6] often referred to as chronological aging, is a gradual and inevitable process that is governed by genetic factors and the natural decline in cellular functions [3,7] over time. On the other hand, extrinsic aging results from environmental influences, such as exposure to ultraviolet [1,8] (UV) radiation, pollution [9], smoking [10], and other lifestyle factors, which accelerate the aging process. The development of wrinkles [11], especially fine and coarse lines around the eyes [12,13], is one of the most visible signs of skin aging. The periorbital area is particularly prone to these changes due to frequent muscle movements, such as blinking, smiling, and frowning, combined with the thinner skin in this region. The periorbital wrinkles are primarily caused by a combination of the repetitive movement of the orbicularis oculi muscles and the loss of collagen and elastin in the skin, both of which contribute to the formation of visible lines. Intrinsic factors such as aging, reduced collagen production, and diminished skin elasticity, as well as extrinsic factors like UV exposure, smoking, and pollution, contribute to this skin degeneration. To address these cosmetic concerns, several treatment options [11,14-16] have been developed, including topical products, Botox injections [17,18], dermal fillers [19,20], and laser treatments [12,21,22], each with varying degrees of effectiveness and safety.
Platelet-Rich Plasma (PRP) [23-25] therapy, a treatment derived from the patient’s own blood, has emerged as a promising alternative for the treatment of periorbital wrinkles. PRP contains a high concentration of growth factors that promote tissue regeneration and collagen production. This therapy has been used to rejuvenate the skin by improving its texture, elasticity, and overall appearance. Studies [26-28] have shown that PRP can effectively reduce fine lines and wrinkles by stimulating collagen production and tissue repair. However, while there is growing evidence supporting the efficacy of PRP in skin rejuvenation, the overall effectiveness of this treatment for periorbital wrinkles remains inconclusive. Given the increasing popularity of PRP in aesthetic medicine, it is crucial to assess its effectiveness compared to other established treatments, such as Botox and dermal fillers. This study aims to conduct a systematic review and meta-analysis of the available literature to evaluate the efficacy and safety of PRP therapy for treating periorbital wrinkles. By synthesizing data from clinical trials, this review seeks to provide clearer insights into the advantages and limitations of PRP, thereby contributing to evidence-based decision-making in aesthetic dermatology.
Materials and Methods
Search strategies
A comprehensive literature search was performed by two reviewers (SS and CS) to identify relevant studies on the use of Platelet-Rich Plasma (PRP) for treating periorbital wrinkles. The search was conducted across multiple electronic databases, including PubMed, Scopus, and The Cochrane Library, covering studies published until August 2024. The search strategy utilized a combination of keywords and Medical Subject Headings (MeSH) terms, including “Platelet Rich Plasma” OR “PRP” combined with terms like “Periocular,” “Periorbital,” “Infraorbital,” “Aging,” “Wrinkles,” and “Crow’s Feet.” The search was restricted to Englishlanguage articles. Additionally, references from relevant reviews and studies were manually searched to identify any other studies that might not have been captured by the database search. The review protocol was registered in PROSPERO (registration number: CRD420251021996).
Study selection
This research was conducted as a systematic review and metaanalysis
following the Preferred Reporting Items for Systematic
Reviews and Meta-Analyses (PRISMA) guidelines [29]. Studies
were included if they:
A. Randomized Controlled Trials (RCTs),
B. included adult participants (aged ≥18 years) with periorbital
wrinkles,
C. investigated the use of Platelet-Rich Plasma (PRP) for treating
periorbital wrinkles,
D. compared PRP therapy to a control group,
E. reported outcomes related to wrinkle reduction or skin
rejuvenation specifically in the periorbital area, using validated
measurement tools. Only studies published in English and appearing
in peer-reviewed journals were considered eligible. Studies were
excluded if they did not investigate PRP treatment, did not report
outcomes related to periorbital wrinkles, or were categorized as
case reports, review articles, or editorials. Additionally, studies that
lacked a control group were excluded. Two independent reviewers
(SS and CS) screened the titles and abstracts of all identified
records for relevance. Full-text articles were retrieved for further
evaluation when they met the inclusion criteria or when eligibility
was unclear. Discrepancies between reviewers were resolved
through discussion, and a third reviewer was involved when
consensus could not be reached.
Data extraction and quality assessment
Data from the included studies were extracted independently by two reviewers using a pre-designed data extraction form. The extracted data included study characteristics (e.g., author, year of publication, study design, sample size), patient demographics (e.g., age, gender), details of the PRP treatment (e.g., dosage, frequency, duration), and outcomes related to the reduction of periorbital wrinkles (e.g., wrinkle severity scores, skin texture improvements). In addition, any adverse effects or complications related to PRP therapy were noted. The quality of the included studies was assessed using the Cochrane Risk-Of-Bias tool (RoB 2) [30] for Randomized Controlled Trials (RCTs). Each study was evaluated across several domains, including random sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and other biases. Studies were categorized as having low, high, or unclear risk of bias. Discrepancies between reviewers were resolved through discussion, and a third reviewer was consulted when necessary.
Statistical analysis
A meta-analysis was conducted to assess the overall effectiveness of PRP therapy in reducing periorbital wrinkles. The primary outcome was the change in wrinkle severity, as measured by validated scales (e.g., Visual Analog Scale, Wrinkle Severity Rating Scale). The pooled Risk Difference (RD) with 95% confidence Intervals (CIs) was calculated for the categorical outcomes. For continuous outcomes, the pooled Mean Difference (MD) or Standardized Mean Difference (SMD) was calculated. Heterogeneity between studies was assessed using the I² statistic, with values of 25%, 50%, and 75% considered to indicate low, moderate, and high levels of heterogeneity, respectively. If significant heterogeneity was present (I² > 50%), a random-effects model was used; otherwise, a fixed-effects model was applied. Sensitivity analysis was conducted to assess the robustness of the results, and publication bias was evaluated using funnel plots and Egger’s test. All statistical analyses were performed using Stata 17 software (StataCorp, College Station, TX, USA) (Table 1).
Table 1:Summary of included studies evaluating the efficacy of PRP treatment compared to control groups.
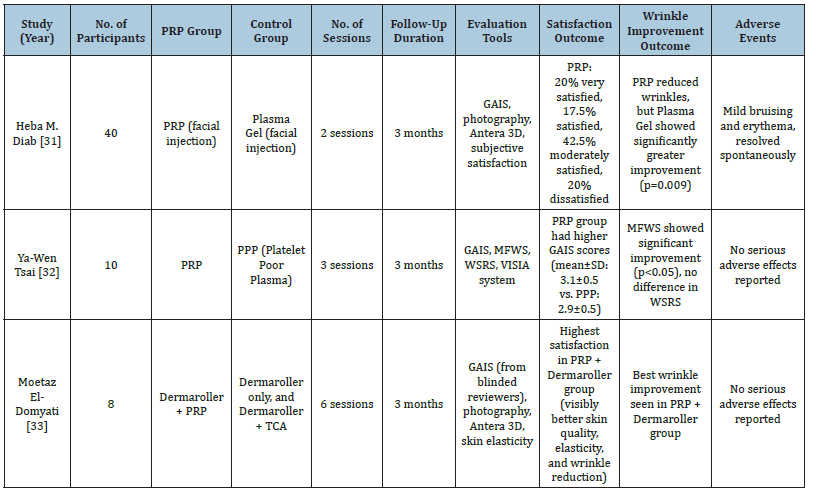
Table Abbreviations: PRP, Platelet-Rich Plasma; PPP, Platelet-Poor Plasma; GAIS, Global Aesthetic Improvement Scale; MFWS, Modified Fitzpatrick Wrinkle Scale; WSRS, Wrinkle Severity Rating Scale; VISIA, VISIA Complexion Analysis System; Antera 3D, Antera 3D Imaging System; TCA, Trichloroacetic Acid.
Result
Search results and study characteristics
Figure 1:PRISMA flow diagram showing the study selection process.
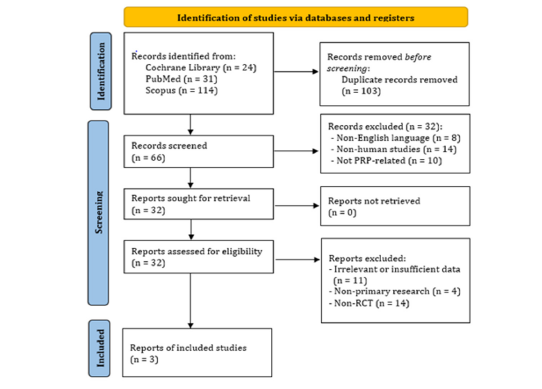
A systematic search was conducted across PubMed, Scopus, and The Cochrane Library for studies published until August 2024 related to Platelet-Rich Plasma (PRP), skin aging, and periorbital wrinkles. A total of 169 records were identified, and 103 duplicate entries were removed, leaving 66 records for initial screening. After reviewing titles and abstracts, 32 records were excluded for the following reasons: non-English language (n=8), non-human studies (n=14), and studies not related to PRP (n=10). Subsequently, 32 full-text articles were assessed for eligibility. Of these, 31 were excluded due to reasons including irrelevant or insufficient data (n=11), non-primary research (n=4), and studies that were not randomized controlled trials (non-RCTs) (n=14). Ultimately, 3 studies [31-33] met all inclusion criteria and were included in the final analysis (Figure 1).
Risk of bias assessment
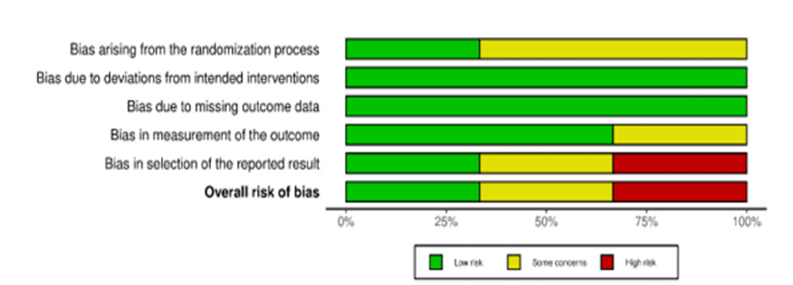
The risk of bias in the included studies was assessed using the RoB 2 tool across five domains. The study by Ya-Wen Tsai [32] exhibited a low risk of bias in all domains, making it the most reliable study among those analyzed. In contrast, the study by Heba M Diab [31] raised some concerns, particularly regarding the randomization process and outcome reporting, as insufficient details were provided about the randomization method and there was no clear evidence of protocol registration, which raised concerns about potential selective reporting. The study by Moetaz El-Domyati [33] was classified as having a high risk of bias, particularly due to unclear outcome reporting and the lack of protocol registration, which suggests the possibility of selective reporting. These varying levels of bias should be considered when interpreting the results of the meta-analysis (Figure 2 & 3).
Figure 2:Risk of bias summary for included randomized controlled trials.
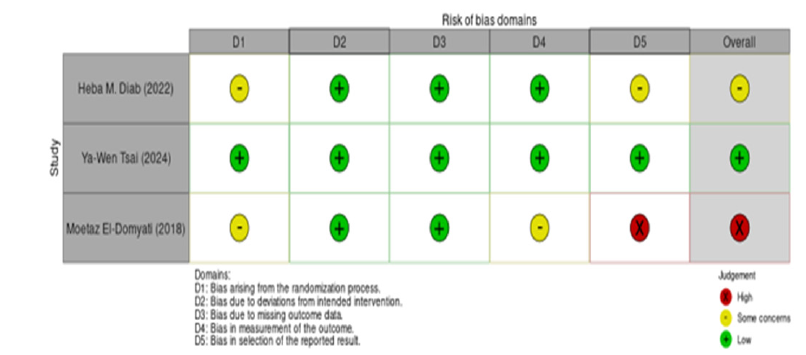
Figure 3:Visual distribution of risk of bias across five domains.
Systematic review results
The systematic review included 3 studies comparing Platelet- Rich Plasma (PRP) with other treatments like plasma gel, Platelet- Poor Plasma (PPP), growth factors, and microneedling with PRP. The studies aimed to assess patient satisfaction, wrinkle outcomes, and adverse effects. All three studies utilized a split-face design, in which PRP was injected on one side of the face while the contralateral side received a control treatment such as plasma gel, PPP, or other agents. The studies involved 8 to 40 female participants, aged 35- 55, with PRP treatment sessions ranging from 2 to 6, and follow-up periods of 1 to 3 months. A variety of outcome assessment tools were used, including the Global Aesthetic Improvement Scale (GAIS), Antera 3D imaging system, VISIA Complexion Analysis System, Modified Fitzpatrick Wrinkle Scale (MFWS), and the Wrinkle Severity Rating Scale (WSRS). PRP generally showed positive results, with 20-50% of patients highly satisfied. GAIS scores for PRP were often higher than control groups, though not always statistically significant. Wrinkle reduction was observed with PRP, though not consistently superior to control treatments. Regarding safety, most studies reported no severe side effects, with temporary swelling, redness, or burning at the injection site. Overall, PRP shows potential for facial rejuvenation, especially for periorbital wrinkles and nasolabial folds, though results depend on the study design and treatment protocols.
Meta-analysis results
The pooled analysis of 3 studies (n=58 participants) using a random-effects model showed no statistically significant difference in patient satisfaction between PRP and control groups, with a risk difference of 0.13 (95% CI: –0.27 to 0.53, p=0.52). Although the point estimate numerically favored PRP, the wide confidence interval and non-significant p-value indicate uncertainty in the true effect. Moreover, the analysis revealed substantial heterogeneity among studies (I²=81.78%, p < 0.001), suggesting that the effect estimates varied considerably across the included trials, thereby limiting the interpretability of the pooled outcome (Figure 4).
Sensitivity analysis (leave-one-out analysis)
Sensitivity analysis using the leave-one-out meta-analysis showed the effect of excluding each study on the overall result. Excluding Heba M Diab’s study [31] led to a significant result (risk difference=0.29, 95% CI: 0.02–0.57, p=0.037), indicating that this study had a significant influence on the overall outcome. In contrast, removing the studies by Ya-Wen Tsai [32] & Moetaz El-Domyati [33] did not show statistically significant results (p > 0.05), indicating minimal impact on the overall result. Thus, Heba M. Diab’s study had a significant influence on the pooled estimate and contributed to the heterogeneity in the meta-analysis. (Figure 5).
Publication bias
Publication bias was assessed using a funnel plot. The plot showed symmetrical distribution of data points within the pseudo 95% confidence interval, suggesting no significant publication bias. While slight asymmetry may be seen, it could reflect variability in study design, sample size, or outcome measures. Given the limited number of studies (<10), no conclusive publication bias was detected.
Discussion
Periorbital wrinkles [11], often referred to as crow’s feet, are among the earliest and most noticeable signs of facial aging. This area is particularly vulnerable due to the thin skin, dynamic muscle activity, and high sun exposure. In recent years, aesthetic interest in autologous therapies has grown substantially, with Platelet- Rich Plasma (PRP) [13,23] emerging as a promising modality for skin rejuvenation. While PRP has been widely adopted in clinical practice due to its perceived safety and biological plausibility, robust evidence supporting its efficacy particularly for periorbital wrinkle reduction remains limited. Previous studies [24,25] have reported variable outcomes depending on patient characteristics, PRP preparation methods, and comparator treatments, raising ongoing debate about its true benefit.
The theoretical foundation for PRP’s use in facial rejuvenation is supported by its autologous composition rich in growth factors [23] such as PDGF, TGF-β, and VEGF. These biomolecules promote dermal regeneration through mechanisms like fibroblast stimulation, angiogenesis, and extracellular matrix remodeling. Such effects are particularly relevant in the periorbital area, where skin aging manifests early. Nonetheless, translating these molecular benefits into visible clinical improvements is complex and depends on multiple variables. Recent evidence also suggests that PRP may exert its anti-aging effects, in part, through the modulation of intracellular signaling pathways associated with longevity and dermal repair. One key molecular target is Sirtuin 1 (SIRT1), a NAD⁺-dependent deacetylase implicated in cellular aging, oxidative stress resistance, and DNA repair [34,35]. Studies have shown that PRP may upregulate SIRT1 expression, thereby enhancing dermal fibroblast function and promoting collagen synthesis [36,37]. SIRT1 activation has also been associated with improved extracellular matrix stability and reduced signs of photoaging, making it a potential mediator of PRP’s clinical efficacy. The interaction between PRP-derived growth factors and SIRT1- regulated pathways warrants further exploration, particularly in the context of periorbital skin where regenerative capacity declines early [34,36].
This systematic review and meta-analysis synthesized data from three [31,32,38] randomized controlled trials (n=58 participants) to evaluate the effectiveness of PRP in improving patient satisfaction and reducing periorbital wrinkles. The overall meta-analysis found no statistically significant difference between the PRP and control groups in satisfaction scores (risk difference=0.13, 95% CI: –0.27 to 0.53, p=0.52). However, sensitivity analysis demonstrated that removing one study [31] yielded a statistically significant pooled effect (risk difference=0.29, 95% CI: 0.02 to 0.57, p=0.037). These results suggest that individual study design, outcome measurement tools, and treatment protocols may substantially influence the pooled estimate.
One key explanation for the non-significant pooled result is the presence of biologically active controls in the included trials, namely Platelet-Poor Plasma (PPP) and plasma gel. These substances, though considered controls, may exert regenerative effects of their own, thereby diminishing the contrast with PRP. In our analysis, this lack of statistical significance may also be attributed to small sample sizes, limited study numbers, and heterogeneity in the control interventions themselves. Additionally, outcome assessments varied across trials, with some studies using subjective scales such as GAIS and satisfaction surveys, while others employed objective imaging tools like VISIA and Antera 3D. This variability likely contributed to inconsistent results and reduced comparability. These findings are in line with prior observations by Gawdat et al. [39] and Redaelli et al. [25], who highlighted inconsistencies related to PRP preparation and application protocols.Despite the mixed efficacy findings, PRP continues to be viewed favorably in clinical settings due to its excellent safety profile, minimal invasiveness, and autologous nature. Across all studies reviewed, adverse events were mild and transient, typically limited to localized erythema, swelling, or bruising that resolved without intervention. This makes PRP particularly attractive for patients seeking non-surgical aesthetic enhancements with low risk. The strengths of this meta-analysis lie in its systematic methodology, use of only RCTs, and inclusion of both subjective and objective assessment tools. To our knowledge, this is one of the few meta-analyses focusing specifically on PRP for periorbital wrinkles. However, limitations remain. The included studies had small sample sizes and follow-up durations limited to 1-3 months, which may not reflect long-term outcomes. Additionally, considerable heterogeneity in PRP preparation protocols (centrifugation force, platelet concentration, injection technique) limits generalizability. The lack of standardized treatment regimens and consistent outcome reporting further impairs data synthesis.
Overall, PRP appears to offer modest benefits for periorbital wrinkle reduction, particularly in selected patient populations. However, its superiority over biologically active comparators remains uncertain. Future studies should adopt standardized PRP protocols and validated outcome measures, ideally with longerterm follow-up and stratified analyses by age, skin type, and wrinkle severity. Further head-to-head comparisons with established treatments such as botulinum toxin, HA fillers, or microneedling will also help clarify PRP’s clinical position in facial rejuvenation.
Conclusion
While PRP showed some positive effects on periorbital rejuvenation, the overall meta-analysis results did not yield statistically significant evidence supporting its superiority over control treatments. The differences observed in individual studies suggest that PRP may have a role in enhancing skin appearance and patient satisfaction, especially in wrinkle reduction. However, due to the heterogeneity of the studies, further large-scale, welldesigned, and standardized trials are needed to confirm its longterm effectiveness and safety.
Acknowledgement
The authors would like to express their sincere gratitude to the staff and faculty of the College of Integrative Medicine, Dhurakij Pundit University, for their academic support and guidance throughout the development of this review. The authors also thank the library services at Dhurakij Pundit University for providing access to essential databases and literature. This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Attia S, Saleh F, Brown D, Birk DE, Gasparro F, et al. (2002) Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Experimental Dermatology 11(5): 398-405.
- Krutmann J, Schikowski T, Morita A, Berneburg M (2021) Environmentally-induced (extrinsic) skin aging: Exposomal factors and underlying mechanisms. J Invest Dermatol 141(4S): 1096-1103.
- Campisi J (1998) The role of cellular senescence in skin aging. J Investig Dermatol Symp Proc 3(1): 1-5.
- Chung JH (2003) Photoaging in Asians. Photodermatol Photoimmunol Photomed 19(3): 109-121.
- Farage MA, Miller KW, Elsner P, Maibach HI (2013) Characteristics of the aging skin. Adv Wound Care 2(1): 5-10.
- Han JM, Ko WS, Yoon HJ (2014) The study on the Korean and western medical literatures for aging and skin aging. The Journal of Korean Medicine Ophthalmology and Otolaryngology and Dermatology 27(1): 45-57.
- Panich U, Sittithumcharee G, Rathviboon N, Jirawatnotai S (2016) Ultraviolet radiation‐induced skin aging: The role of DNA damage and oxidative stress in epidermal stem cell damage mediated skin aging. Stem Cells Int: p. 7370642.
- Gonzaga ER (2009) Role of UV light in photodamage, skin aging, and skin cancer: Importance of photoprotection. Am J Clin Dermatol 10(Suppl 1): 19-24.
- Puri P, Nandar SK, Kathuria S, Ramesh V (2017) Effects of air pollution on the skin: A review. Indian J Dermatol Venereol Leprol 83(4): 415-423.
- Morita A (2007) Tobacco smoke causes premature skin aging. J Dermatol Sci 48(3): 169-175.
- Porada H, Bouougri EH (2007) Wrinkle structures-A critical review. Earth-Science Reviews 81(3-4): 199-215.
- Barikbin B, Akbari Z, Vafaee R, Razzaghi Z (2019) The efficacy of IPL in periorbital skin rejuvenation: An open-label study. J Lasers Med Sci 10(Suppl 1): S64-S67.
- Reed JT, Joseph AK, Bridenstine JB (1997) Treatment of periorbital wrinkles. A comparison of the silktouch carbon dioxide laser with a medium-depth chemical peel. Dermatol Surg 23(8): 643-648.
- Kadhim KA, Waiz MA (2005) Treatment of periorbital wrinkles by repeated medium‐depth chemical peels in dark‐skinned individuals. J Cosmet Dermatol 4(1): 18-22.
- Trelles MA, Pardo L, Benedetto AV, Solana LG, Torrens J (2000) The significance of orbital anatomy and periocular wrinkling when performing laser skin resurfacing. Dermatol Surg 26(3): 279-286.
- Turlier V, Rouquier A, David B, Josse G, Auvergnat A, et al. (2010) Assessment of the clinical efficacy of a hyaluronic acid-based deep wrinkle filler using new instrumental methods. J Cosmet Laser Ther 12(4): 195-202.
- Carruthers J, Alastair C (2003) Aesthetic botulinum A toxin in the mid and lower face and neck. Dermatol Surg 29(5): 468-476.
- Cheng CM (2007) Cosmetic use of botulinum toxin type A in the elderly. Clin Interv Aging 2(1): 81-83.
- Jennen D, Keizers PHJ, Hodemaekers HM, Vermeulen JP, Bakker F, et al. (2022) Evaluation of adverse effects of resorbable hyaluronic acid fillers: Determination of macrophage responses. International Journal of Molecular Sciences 23(13): 7275.
- Kyriazidis I, Spyropoulou GA, George Z, Tagka A, Gasteratos K, et al. (2024) Adverse events associated with hyaluronic acid filler injection for non-surgical facial aesthetics: A systematic review of high level of evidence studies. Aesthetic Plast Surg 48(4): 719-741.
- Henry HLC, Lam Lk, Wong DSY, Kono T, Nigel TS (2004) Use of 1,320nm Nd: YAG laser for wrinkle reduction and the treatment of atrophic acne scarring in Asians. Lasers in Surgery and Medicine 34(2): 98-103.
- Chen KH, Tam KW, Huang SK, Tzeng PC, Wang HJ, et al. (2017) A systematic review of comparative studies of CO2 and erbium: YAG lasers in resurfacing facial rhytides (wrinkles). J Cosmet Laser Ther 19(4): 199-204.
- Gentile P, Garcovich S (2020) Systematic review-the potential implications of different platelet-rich plasma (PRP) concentrations in regenerative medicine for tissue repair. Int J Mol Sci 21(16): 5702.
- Ismail A, Reynolds KA, Poon E, Serrano L, Grushchak S, et al. (2020) A systematic review of the safety and effectiveness of platelet-rich plasma (PRP) for skin aging. Arch Dermatol Res 312(5): 301-315.
- Redaelli A, Domenico R, Antonio M (2010) Face and neck revitalization with Platelet-rich plasma (PRP): Clinical outcome in a series of 23 consecutively treated patients. J Drugs Dermatol 9(5): 466-472.
- Chahla J, Cinque ME, Mannava S, Geeslin AG, Murray IR, et al. (2017) A call for standardization in platelet-rich plasma preparation protocols and composition reporting: A systematic review of the clinical orthopaedic literature. J Bone Joint Surg Am 99(20): 1769-1779.
- Cho JM, Lee YH, Baek RM, Lee SW (2011) Effect of platelet-rich plasma on ultraviolet b-induced skin wrinkles in nude mice. J Plast Reconstr Aesthet Surg 64(2): e31-e39.
- Marx RE (2004) Platelet-rich plasma: Evidence to support its use. J Oral Maxillofac Surg 62(4): 489-496.
- Page MJ, Boutron I, Bossuyt PM, Hoffmann TC, Mulrow CD, et al. (2021) The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372: n71.
- Minozzi S, Cinquini M, Gianola S, Marien GL, Banzi R (2020) The revised cochrane risk of bias tool for randomized trials (RoB 2) showed low interrater reliability and challenges in its application. J Clin Epidemiol 126: (37-44).
- Diab HM, Elhosseiny R, Bedair NI, Khorkhed AH (2022) Efficacy and safety of plasma gel versus platelet-rich plasma in periorbital rejuvenation: A comparative split-face clinical and antera 3D camera study. Arch Dermatol Res 14(7): 661-671.
- Tsai YW, Cheng CY, Chang SL, Lin TM, Huang YL, et al. (2024) Platelet-rich plasma versus platelet-poor plasma for treating facial photoaging: A double-blind randomized controlled splitting face study. Aesthetic Plast Surg 48(11): 2162-2170.
- Moetaz ED, Hossam AW, Hossam A (2018) Combining microneedling with other minimally invasive procedures for facial rejuvenation: A split‐face comparative study. Int J Dermatol 57(11): 1324-1334.
- Martins IJ (2016) Anti-aging genes improve appetite regulation and reverse cell senescence and apoptosis in global populations. Advances in Aging Research 5(1): 9-26.
- Martins I (2017) Nutrition therapy regulates caffeine metabolism with relevance to NAFLD and induction of type 3 diabetes. J Diabetes Metab Disord 4(1): 100019.
- Martins I (2018) Sirtuin 1, a diagnostic protein marker and its relevance to chronic disease and therapeutic drug interventions. EC Pharmacology and Toxicology 6(4): 209-215.
- Weng HP, Cheng YY, Lee HL, Chang YT, Shen YA, et al. (2021) Enhanced platelet-rich plasma (ePRP) stimulates wound healing through effects on metabolic reprogramming in fibroblasts. Int J Mol Sci 22(23): 12623.
- Domyati ME, Hossam AW, Hossam A (2018) Microneedling combined with platelet‐rich plasma or trichloroacetic acid peeling for management of acne scarring: A split‐face clinical and histologic comparison. J Cosmet Dermatol 17(1): 73-83.
- Gawdat HI, Tawdy AM, Hegazy RA, Zakaria MM, Allam RS (2017) Autologous platelet‐rich plasma versus readymade growth factors in skin rejuvenation: A split face study. Journal of Cosmetic Dermatology 16(2): 258-264.
© 2025 Sirilak Sutthinont. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






