- Submissions

Full Text
Novel Research in Sciences
Development and Characterization Studies of Emulgel for the Topical Delivery of 5-Fluorouracil for the Treatment of Skin Cancer
Deepti Dubey1, Manoj Kumar Mishra2*, Vimal Yadav3 and Ajay Kumar Shukla3
1Daksh Institute of Pharmaceutical Science, Chhatarpur, Madhya Pradesh, India
2Shambhunath Institute of Pharmacy, Jhalwa, Prayagraj, Uttar Pradesh, India
3Institute of Pharmaceutical Sciences, Dr. Rammanohar Lohia Avadh University, Ayodhya, Uttar Pradesh, India
*Corresponding author:Manoj Kumar Mishra, Shambhunath Institute of Pharmacy, Jhalwa, Prayagraj, Uttar Pradesh, India
Submission: March 06, 2023;Published: March 15, 2023
.jpg)
Volume14 Issue2March , 2023
Abstract
Background: 5-Fluorouracil (5-FU) is one of the most effective antineoplastic drug used for the treatment
of actinic keratosis and nonmelanoma skin cancer. It shows poor percutaneous permeation through the
conventionally available forms of solution and cream with shorter retention time is inefficient for the
treatment of deep-seated skin cancer.
Objective: In the present study, emulgel containing 5-FU was investigated for the treatment of skin cancer.
Methods: Different formulations of emulgel were prepared using Tween-20, Span-20, liquid paraffin,
different oils like clove oil and Mentha oil.
Result: The formulations were evaluated for parameters like optical microscopy, drug content, viscosities,
bio adhesive strength, stability studies, in-vitro drug release, skin deposition and interaction study.
Optimized formulations, F2 and F1 were selected in term of maximum drug release, drug entrapment
values for higher studies. Mentha oil with 4%(F1) and 6% (F2 v/v showed excellent physicochemical
properties such as viscosity (1300 and 1400 Cp), bio adhesive strength (4.3±0.13 and 5±0.21kg/cm2), invitro
release (56.01 and 53.48 %). Stability study for one month puts a slight deterioration in drug content.
Conclusion: The result of the present study demonstrated that formulation of Mentha oil based 5-FU bio
adhesive gel is a better alternative to the traditional cream base for enhanced topical delivery of 5-FU.
The developed formulation will have the ease of application, better skin deposition and sustained release
characteristic with reduced skin toxicity.
Keywords:5- Fluorouracil; Emulgel; Skin cancer; Carbopol 940; Clove oil; Mentha oil
Introduction
Skin cancer is the most common type of cancer affecting Caucasian populations [1]. It has a very high rate of incidence, exceeding the sum of all other cancers combined. Melanoma skin cancers are an aggressive type that can metastasize and cause death [2]. These cancers originate from melanocytes, which are pigment-producing cells, and are associated with chronic exposure to sunlight [3]. Treatments for melanoma, in turn, are primarily surgical because these tumors can be resistant to traditional chemo- and radiotherapies [4]. The best way to overcome the limitation of conventional drug delivery system is the use of different carrier systems like liposomes, noisome, Ethosomes, elastic liposome’s, micro emulsion, solid lipid Nano particles and nano-structured lipid carrier or some alternate cost-effective system like emulgel or organogold [5]. There is a need for effective delivery systems that not only act as a formulation aid but also alter the biodistribution of drugs in such a way that a greater fraction of dose reaches the target site and improves the local bioavailability of the drug. In the present study, it is proposed to develop the emulgel based topical formulation for skin targeting of anti-cancer drug.
Preformulation Study
Organoleptic properties, melting point and calibration curve of curcumin was prepared. The purpose of the study was to generate information valuable for the preparation of stable and bioavailable dosage form. Preformulation studies are needed to ensure the development of a stable as well as therapeutically effective and safe dosage form. Characterization of various physicochemical properties of drug substances like physical appearance, melting point, chemical identification, solubility, and partition coefficient.
Physical appearance
The drug (5-FU) powder was white crystalline powder.
Melting point
Melting point of 5-FU was determined by melting point apparatus (Tempo Instruments Pvt. Ltd., China) and found to be 280-284 °C.
Chemical test for identification:
5mL of 1% w/v bromine solution was prepared in distilled water and few crystals of 5- FU was added to it which decolorized the bromine water.
Solubility
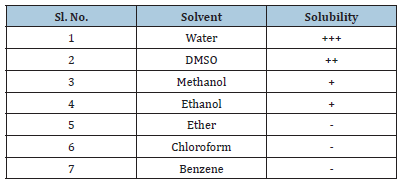
The sample was qualitatively tested for its solubility in various solvents. An equivalent amount (1g) of drug was taken in separating flasks containing 5mL of solvents (water, methanol, ethanol, ether, chloroform, and benzene) using a mechanical shaker at 25 °C for 24h. Samples were centrifuged (2000rpm), membrane filtered (#5mm) and the solubility of the drug in solvents were analyzed by UV-Visible spectroscopy (Table 1).
Table 1:Solubility of 5-FU in different solvents.
++++=Freely soluble
+++=Sparingly soluble
++=Soluble
+=Slightly soluble
–=Practically insoluble
Partition coefficient
The partition behavior of drug was examined in n-Octanol: water, n-Octanol: PBS (pH 6.8), and n-Octanol: PBS (pH 7.4) systems. It was determined by taking 10mg of drug in three separating funnels containing 10mL portions of each n-Octanol and 10mL water, 10mL of PBS (pH 6.8) and 10mL of PBS (pH 7.4). The separating funnels were shaken for 24hrs in a wrist action shaker for equilibration. Two phases were separated and the amount of the drug in aqueous phase was analyzed spectrophotometrically (Shimadzu UV-2450, Dongguan, China) at 266.0nm after appropriate dilution. The partition coefficient of the drug in phases was calculated by using following formula [6] (Table 2):
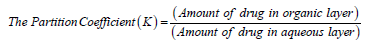
Table 2:Partition coefficient values of 5-FU.
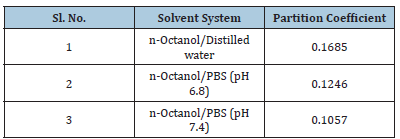
Determination of interference of polymers in the estimation of 5-FU
Accurately weighed quantity of 5- FU (10mg) was dissolved in 10mL of various solvents in 100mL of volumetric flask. To each flask 10mg of additives were dissolved in distilled water and allowed to equilibrate for 2h. It was filtered and diluted with distilled water. The absorbance was noted at 266.0nm. The observations are recorded (Table 3).
Table 3:Absorbance data for interference of additives in the estimation of 5-FU.
Preparation and Characterization of Emulgel
Preparation of 5-FU formulation
Different formulations were prepared using varying amount of gelling agent and penetration enhancers. The method only differed in process of making gel in different formulation. The gel phase in the formulations was prepared by dispersing Carbopol 940 in purified water with constant stirring at a moderate speed using mechanical shaker then the pH was adjusted 6-6.5 using triethanolamine (TEA). The oil phase of the emulsion was prepared by dissolving span 20 in the light liquid paraffin while aqueous phase was prepared by dissolving tween 20 in purified water. Methyl and propyl parabens were dissolved in propylene glycol whereas 5-FU was dissolved in ethanol and solutions were mixed with the aqueous phase. Mentha oil and clove oil were mixed in oil phase. Both the oily and aqueous phases were separately heated to 70-80 °C, then oily phase was added to aqueous phase with continuous stirring until it got cooled to the room temperature. The obtained emulsion was mixed with the gel in 1:1 ratio with gentle stirring to obtain the emulgel [7]. The composition of different formulations has been discussed (Table 4).
Table 4:Composition of different formulation batches.
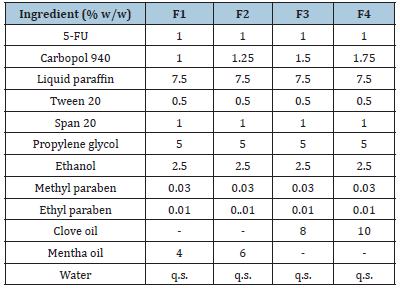
Characterization of drug-loaded emulgel preparation
In-vitro release studies:The in-vitro drug release studies were carried out using modified Franz diffusion cell [8]. The formulation was applied on dialysis membrane which was placed between donor & receptor compartment of the Franz Diffusion cell. Phosphate buffer pH 7.4 was used as dissolution media. The temperature of the cell was maintained at 37 °C by circulating water jacket. This assembly was kept on magnetic stirrer and the solution was stirred continuously using a magnetic bead. A similar blank set was run simultaneously as a control. The 5mL sample was withdrawn at suitable time intervals and replaced with equal amount of fresh dissolution media. Samples were analyzed spectrophotometrically at 285nm and the cumulative percentage drug release was calculated. The difference between the reading of drug release and control was used as the actual reading in each case.
Physical examination:The prepared emulgel formulations were inspected visually for their consistency, grittiness, color, homogeneity and phase separation.
Drug content [9]:Weigh accurately 1g of emulgel and it was dissolved in 100mL of phosphate buffer 6.8. The volumetric flask was kept for 2h and shaken well in a shaker to mix it properly. A solution was passed through filter paper and the filtered. The absorbance measured spectrophotometrically after appropriate dilution against corresponding emulgel concentration as blank.
Measurement of pH:The pH of emulgel formulations was determined by the using of digital pH meter (CK1, Systolic, China). 1g of gel was dissolved in 100mL of the distilled water and it was placed for 2h. The measurement of pH of each formulation was done in triplicate and average values were calculated.
Determination of viscosity:Viscosities of the formulated batches were determined by using a Brookfield viscometer. The formulation whose viscosity was to be determined [10]. Added in the beaker and was allowed to settling down for 30min at the assay temperature before the measurement was taken. Spindle was lowered perpendicular into the centre of emulgel taking care that spindle does not touch bottom of the jar and rotated at speed 12rpm for 10min. The viscosity reading was noted.
Spreadibility:One of the criteria for emulgel to meet the ideal quantities is that it should possess better spreadibility. It is the term expressed to denote the extent of area to which gel readily spreads on application to the affected part of the skin [11]. The therapeutic efficacy of the formulation also depends upon its spread ability of emulgel and marketed gel was measured in terms of diameter of emulgel circle produce when emulgel is placed between two glass plates of definite weight [12]. A weight quantity (350mg) of emulgel was taken on one glass plate and another plate was dropped from a distance of 5cm. The diameter of the circle of spread emulgel was measured.
Bio adhesive strength measurement:The bio adhesive strength is maximum adhesive force between the bio adhesive and targeted substrate must be strong enough to ensure that an effective concentration of the drug is maintained at the specific site for a sufficient time period. In this test, bio adhesive layer was applied to flat surface of the upper specimen holder and fixed to the movable crosshead of with 10N load cell. It was then brought into contact with the targeted substrate and attached to the lower specimen holder. A preload of 5N was applied and maintained for 6sec to allow for formation of adhesion bonds. After the holding period, the crosshead was moved upward at constant speed to separate the two surfaces and maximum adhesion force was recorded. The test was performed under physiological conditions using a custom temperature-controlled Bath. Bath allows accurate control of the bath temperature at 37±1 °C [13].
Stability study:Stability of any pharmaceutical product is usually defined as the capacity of the formulation to remain within defined limits over a predetermined period of time (shelf life of the product). The World Health Organization (WHO), International Conference on Harmonization (ICH) and the Food and Drug Administration (FDA) has published general guidelines pertaining to the assessment of stability of drug substances and/ or drug products. The guidelines were used as a template to design a stability protocol for use in the assessment of stability of the Acyclovir topical emulgel and ethel based formulation developed in this study. Stability studies were conducted using a low temperature incubator (Narang Scientific Pvt, Ltd.). A week prior to use, the incubator was allowed to equilibrate to the temperature of 40±5 °C and relative humidity of 75±5%. The collapsible tubes containing 10g of cream were placed in the equilibrated incubator and at weeks 1, 2, 3 and 4 sample was removed from the incubator and immediately analyzed in terms of the test parameters given below.
The qualitative parameters evaluated for the test formulations
include:
A. Appearance
B. Colour
C. Odour
D. Signs of physical instability
The quantitative parameters evaluated for the test formulations
include:
a) Drug content
b) Intrinsic viscosity
c) Intrinsic pH
The results of stability study are summarized in table. Developed emulgel formulation was found to be stable in accelerated conditions of stability testing.
Results and Discussion
An infrared spectrum of provided drug was found to be concordant with the reference infrared spectrum of the 5-FU [14]. Solubility study in different solvents at room temperature revealed that it is soluble in distilled water and insoluble in chloroform, benzene etc. Partition coefficient value of 5-FU also revealed its hydrophilic nature {PO/W = 16.85x10-2 for n-Octanol/ water, 12.96x10-2 for n-Octanol/PBS (pH 7.4) and 10.57x10-2 for n-Octanol/PBS (pH7.6)}. The absorbance data of both drug and different additives were noted. The absorbance data had shown no appreciable change in the absorbance of drug solon at 266.0nm indicating no interference of polymers in the estimation of 5- FU.
Physical appearance:
Table 5:Physical parameters of formulation batches.
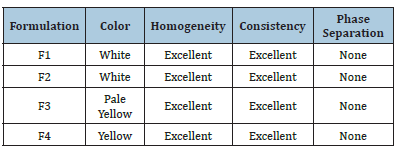
The emerges were assessed physically and visually for color (white viscous creamy), and consistency. Emulgel formulations were yellowish white viscous creamy preparation with a smooth homogeneous texture, good appearance, and glossy appearance. Results have been discussed in Table 5.
Morphological appearance (Optical microscopy)
Emulgel was viewed under light microscope to study the globular structure in gel base. The emulgel was suitably diluted, mounted on glass slide and viewed by light microscope under magnification of 40x. Results have been discussed in Figure 1 (Table 6). Viscosity is an important parameter for a topical drug delivery system. It affects drug release and absorption at the site of action and thus affects the therapeutic benefit from the formulation [15]. Emulgel formulations with Carbopol had good viscoelastic properties, and their formulations had the possibility of returning to their original state once the stress is removed [16]. In the present study, we observed that viscosity increases in the percentage of polymer (Carbopol 940) led to a viscous emulgel. A spread ability test was performed for all the formulations, and the spread ability was found to be in the range of 10±0.21 to 14±0.62. The spread ability of the emulgel formulations decreased with an increase in the concentration of the polymer. The spread ability is important because it indicates how the emulgel will behave when it is extruded from the tube.
Figure 1: Morphological appearance using optical microscopy.
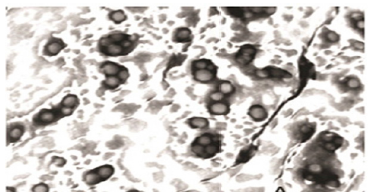
Table 6:Various factors associated with formulations.
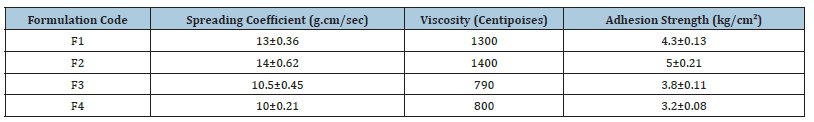
(n= 3, all data represents the mean ±SD value).
In-vitro drug release study
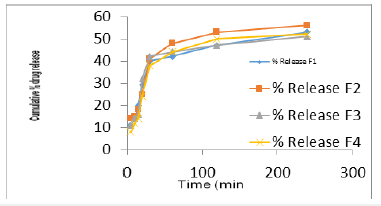
The therapeutic efficacy of any drug is dependent upon the release of drug from pharmaceutical preparation [17]. The release of drug from topical formulation depends upon several factors including gelling agents (several polymers), emulsifying agents (surfactants used), spread ability, and viscosity [18]. The study showed the release of the drugs from its emulsified gel formulation can be ranked in the following descending order: F2>F1>F4>F3 where the amounts of the drug release of the drug released after 240min were 56.01%, 53.48%, 52.23%, 51.21%, respectively. The drug released depended on the concentration of polymer; an increase in the concentration of polymer caused the drug release time to decrease and the diffusion through the membrane to decrease [19] (Figure 2).
Figure 2: In-vitro cumulative % drug release of formulation F1-F4; The data were presented as the mean ± SD and were analyzed using ANOVA p<0.05 refers to statistical significance.
Optimization of formulation
From the result formulation F2 and F1 show maximum drug release, drug entrapment, spread ability, viscosity and bioderived strength. So, these formulations were selected for further study.
Drug content
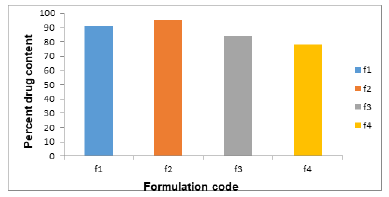
Drug content details of emulgel are shown (Figure 3).
Figure 3:% drug content of formulation F1-F4.
Stability Studies
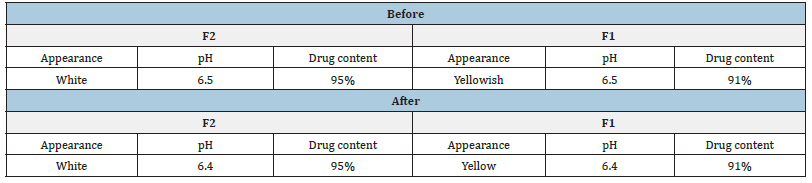
A stability study was performed on optimized batches F2 and F1 in ambient conditions. The results obtained after 1 month time period are shown in Table 7.
Table 7:Stability studies of the optimized formulations.
Conclusion
As the emulgel is recent technique for topical drug delivery it is better suitable for hydrophobic drugs and it is very good technique for drug delivery of combination of both hydrophilic and hydrophobic drugs. Since emulgel had appear as a new and novel technique for topical drug delivery. Mainly the hydrophobic drug formulation can be developed with emulgel technique on the other hand hydrogel are not suitable for hydrophobic drugs. In the present article, emulgel containing 5-FU was investigated for the treatment of skin cancer. Different formulation of emulgel was prepared using Tween-20 and Span-20 as edge activators. The vesicles were characterized for shape, particle size, percentage entrapment efficiency, deformability and In- Vitro skin permeation. Optimized formulation was incorporated into Carbopol 940gel and evaluated for efficacy in the treatment of skin cancer. We concluded that the developed 5-FU-loaded transpersonal gel improves the skin absorption of 5-FU and provide a better treatment for skin cancer applications. In addition, low-dose 5-FU appeared to have good general tolerability and was well accepted by both physicians and patients in terms of clinical and cosmetic outcomes. In the coming years, topical drug delivery will be used for extensively to impart better patient compliance. Since emulgel is helpful in enhancing spread ability, adhesion, viscosity and extrusion, this novel drug delivery become popular, more ever they will become a solution for loading hydrophobic drugs in water soluble gel base for the longterm stability. The formulations F1 and F2 were comparable with marketed topical gel. So, 5-fluorouracil emulgel can be used as an anti-cancer agent for topical drug delivery.
References
- Ridky TW (2007) Non-melanoma skin cancer. Journal of the American Academy of Dermatology 57(3): 484-501.
- Zbytek B, Carlson JA, Granese J, Ross J, Mihm MC, et al. (2008) Current concepts of metastasis in melanoma. Expert Rev Dermatol 3(5): 569-585.
- Brenner M, Hearing VJ (2008) The protective role of melanin against UV damage in human skin. Photochem Photobiol 84(3): 539-549.
- Davis LE, Shalin SC, Tackett AJ (2019) Current state of melanoma diagnosis and treatment. Cancer Biol Ther 20(11): 1366-1379.
- Hassan DH, Shohdy JN, El Setouhy DA, El Nabarawi M, Naguib MJ (2022) Compritol-based nanostructured lipid carriers (nlcs) for augmentation of zolmitriptan bioavailability via the transdermal route: In vitro optimization, ex vivo permeation, in vivo pharmacokinetic study. Pharmaceutics 14(7): 1484.
- Bannan CC, Calabró G, Kyu DY, Mobley DL (2016) Calculating partition coefficients of small molecules in octanol/water and cyclohexane/water. J Chem Theory Comput 12(8): 4015-4024.
- Jagdale S, Pawar S (2017) Gellified emulsion of ofloxacin for transdermal drug delivery system. Adv Pharm Bull 7(2): 229-239.
- Salamanca CH, Barrera OA, Lasso JC, Camacho N, Yarce CJ (2018) Franz diffusion cell approach for pre-formulation characterisation of ketoprofen semi-solid dosage forms. Pharmaceutics 10(3): 148.
- Burki IK, Khan MK, Khan BA, Uzair B, Braga VA, et al. (2020) Formulation development, characterization, and evaluation of a novel dexibuprofen-capsaicin skin emulgel with improved in vivo anti-inflammatory and analgesic effects. AAPS Pharm SciTech 21(6): 1-14.
- Monica R, Girish S, Sheetal A, Manmeet K (2013) Optimization of metronidazole emulgel. Journal of Pharmaceutics 2013: 501082.
- Rachit K, Saini S, Seth N, Rana AC (2011) Emulgels: A surrogate approach for topically used hydrophobic drugs, Int J Pharm Bio Sci 1(3): 117-128.
- Khan BA, Ullah S, Khan MK, Alshahrani SM, Braga VA (2020) Formulation and evaluation of Ocimum basilicum based emulgel for wound healing using animal model. Saudi Pharm J 28(12): 1842-1850.
- Young CS, Sah H, Jahng Y, Chang HW, Son JK, et al. (2003) Physiochemical characterization of diclofenac sodium loaded poloxamer gels as a rectal delivery system with fast absorption. Drug Dev Ind Pharm 29(5): 545-553.
- Giambattista DL, Pozzi D, Grimaldi P, Gaudenzi S, Morrone S, et al. (2011) New marker of tumor cell death revealed by ATR-FTIR spectroscopy. Anal Bioanal Chem 399(8): 2771-2778.
- Khan BA, Khan A, Khan MK, Braga VA (2020) Preparation and properties of high sheared poly (vinyl alcohol)/chitosan blended hydrogels films with Lawsonia inermis extract as wound dressing. J Drug Deliv Sci Technol 61: 456-462.
- Rohatagi S, Gillen M, Aubeneau M, Jan C, Pandit B, et al. (2001) Effect of age and gender on the pharmacokinetics of ebastine after single and repeated dosing in healthy subjects. Int J Clin Pharmacol Ther 39(3): 126-134.
- Deng Y, Liu Z, Geng Y (2016) Anti-allergic effect of artemisia extract in rats. Exp Ther Med 12(2): 1130-1134.
- Islam N, Irfan M, Zahoor AF, Iqbal MS, Syed HK, et al. (2021) Improved bioavailability of ebastine through development of transfersomal oral films. Pharmaceutics 13(8): 1315.
- Kumar V, Mahant S, Rao R, Nanda S (2014) Emulgel based topical delivery system for loratadine. ADMET and DMPK 2(4): 254-271.
© 2023 Manoj Kumar Mishra. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






