- Submissions

Full Text
Novel Research in Sciences
Effect of Feeding on Bovine Milk Quality
Miguel Ángel Galina1*, Rosa Isabel Higuera-Piedrahita1, Sebastián Carrillo1, Nadia Musco2, Jorge Pineda3, Pedro Vázquez4, George Haenlein5, Federico Infascelli2 and Jorge Olmos6
1Faculty of Higher Studies Cuautitlán, National Autonomous University of Mexico, Mexico
2Department of Veterinary Medicine and Animal Production, University of Napoli Federico II,Italy
3Faculty of Veterinary Medicine and Zootechnics, University of Colima, Mexico
4Center for Research in Applied Science and Advanced Technology National Polytechnic Institute,Queretaro, Mexico
5Department of Animal & Food Sciences, University of Delaware, USA
6Faculty of Veterinary Medicine and Zootechnics Autonomous, University of Querétaro, Mexico
*Corresponding author: Miguel Ángel Galina, Faculty of Higher Studies Cuautitlán, National Autonomous University of Mexico, Mexico
Submission: October 15, 2022;Published: October 21, 2022
.jpg)
Volume12 Issue3October , 2022
Abstract
The trial aimed to compare the fatty acid profile of milk from dairy cows under Exclusive Grazing (EG), Supplemented Grazing (SG) or Full Confinement (FC) system. Sampling was performed in 2017 and 2018 on 6707 dairy cows from Querétaro, Tabasco, Colima, Veracruz and Chiapas in Mexico. Among the 84 farms included in the trial, 25.3% were in FC (1699 animals) and fed corn silage, alfalfa hay, tropical forages and commercial balanced concentrate (16% CP; 5 to 7kg/ head/day); in both extensive (EG) and Supplemented (SG) Grazing there was a mixture of tropical grasses: Cynodon niemfluensis, Muhlenbergia robusta, Brachiaria brizantha, B. decumbens and Echinochloa Polystachya. Group EG (30%, 2014 heads) was permanently grazing while SG (44.6%, 2944 heads) was also supplied with a commercial balanced feed (18% crude protein; 2 to 3kg/head/day). Average daily milk yield was significantly (P<0.05) different among groups: 16.2±2.12kg (FC), 9.5±2.72kg (SG) and 7.2±1.530kg (EG). The breeding system also affected milk fatty acid profile, particularly the ω6/ω3 ratio: increasing the amount of concentrate in the diet significantly (P<0.05) increased milk ω6 or decreased ω3 concentration, thus diminishing the beneficial effects for human health.
Keywords:Milk; Grazing; PUFA; Omega fatty acids; Omega6/Omega3 ratio
Introduction
In recent years, the consumers demand for foods with high nutritional value has strongly increased [1]. Concerning those of animal origin, it is accepted that animal diet can affect their quality [2-5]; in particular, foods produced by grazing ruminants are recognized by nearly all consumers, and farmers themselves, as high-quality foods [6]. On the other hand, dairy specialization and intensive farming have brought about an increase in the use of concentrates, thus reducing or even eliminating pasture as a feed source in many countries [7]. Indeed, in some areas like the tropics, there is still an abundant source of natural grasses and leguminous trees, that allows to feed cows in silvopasture environments, thus producing high quality milk [8]
Previous studies on the nutritional quality of milk in Mexico demonstrated several benefits of grazing in Zebu cow and goat [8]. In fact, lower content of Saturated Fatty Acids (SFA) and higher levels of ω3 Polyunsaturated Fatty Acids (PUFA), were observed in milk from grazing animals compared to that from animals in full confinement [9,10]. It has been proven that a lower content of SFA favors human health [6], as well as it has been demonstrated that ω3 PUFAs, in Particular Arachidonic Acid and Docosahexaenoic Acid (DHA), are able to improve oxidative stress. This is critical, since oxidative stress is characterized by a decrease in the capacity of the endogenous system to act against oxidative attack directed to biomolecules, and it has been associated with different severe pathologies, such as cancer, cardiovascular diseases, type 2 diabetes, hypertension, and neurodegenerative diseases [6].
Other researches focused on the importance of ω6/ω3 ratio [11], suggesting a value of 4 may prevent cardiovascular diseases up to a 70% reduction in mortality. More recently, the importance of maintaining a ω6/ω3 ratio lower than 4 was underlined [12,13] also because, in modern diets it results higher than 10 [14,15]. Thus, milk from grazing ruminants could have beneficial effects of human health, contributing to decrease the ω6/ω3 ratio of the diet. Aim of this study was to evaluate, over two years, the effect of grazing on the milk fatty acids profile, particularly the ω6/ω3 ratio, by comparing 6707 lactating cows undergoing three different breeding systems in Mexico.
Materials and Methods
Experimental design and treatments
The experiment was performed on bulk milk of 84 farms (total of 6707 dairy cows, 2nd to 4th parity) in Mexico, for two years: 2017 and 2018. The farms were in Querétaro (Latitude: 20.5931, Lenght: -100.392 20°, at 1820msnm), Tabasco (18°20’ north latitude, 93°15’ length, 10 meters over the sea), Colima (latitude 19.2433, lenght-103.725 19° 14′ 36″ North, 103° 43′ 30″ West, over 550msnm), Veracruz (17° 09´ latitude, 98°39´ length, 0 meters over the sea) and Chiapas (lenght: O93°22’51.74” latitude: N17°33’23.51”, at 4080msnm). In each farm, the calving’s were grouped, thus, most of the cows had the same days in milk; the lactation stages considered were from 30 to 60 days postpartum and 90 to 110 days postpartum.
Feeds and milk sampling and analysis
In May and August of each year, bulk tank milk samples (100mL) were collected once a day for three consecutive days, in a sterilized plastic falcon tube, refrigerated at 3 °C and transported to the laboratory. An aliquot of each sample was analyzed for fat, protein and lactose (MilkoScan™ 133B, Fos Matic, Hilleroed, Denmark) while another one was refrigerated at 3 °C for 4h, frozen at -21 °C for 48h and then lyophilized.
Contemporary, in EG and SG system pasture samples were collected as follows: grass of fou different areas (2.5m2 each) was cut at 3cm from the ground; once weighed, 4 representative samples (1kg each, obtained balancing the amount from the 4 different areas) were air-oven dried at 65°C, milled through a 1mm screen and analysed according to AOAC [16] for dry matter (DM, ID 934.01), crude protein (CP, ID 984.13), ether extract (EE, ID 920.29); the structural carbohydrates were also determined [17] and nutritive value (UFL=1700kcal of net energy for lactation) was calculated [18-33].
Milk fatty acid analyses
Total fat of milk lyophilized samples was separated by a mixture of hexane isopropane (3/2,v/v), according to Hara A, et al. [34]. Transmethylation of fatty acids was performed by the base-catalysed procedure described by Christie WW, et al. [19] and modified by Chouinard PY, et al. [20]. FAME were quantified by Gas Chromatography (GC) using a CP-3380 chromatograph equipped with a split injector, Flame Ionization Detector (FID) and auto sampler CP 8400. A DB23 column (30m x 0.25mm i.d.) with a film thickness of 0.25μm was employed. Nitrogen was used as carrier gas at a flow rate of 30ml/min. Temperatures column was held for 1 min at 120°C, then programmed at rate of 10°C/ min to 200°C and held for 5°C/min to final temperature of 230°C; temperature injector and FID were 250°C and 300°C, respectively. Integration for each fatty acid was performed by a Varian Star Chromatography Workstation Software. Identification of the peaks was made on the basic of the retention times of standard methyl esters of individual fatty acid (FAME mix C4-C24 #18919-1 AMP). The finalconcentration of FAME was expressed as mg/100 g of milk.
Atherogenic and Thrombogenic index
To better characterize the milk nutritional characteristics, the Atherogenic Index (AI) and Thethrombogenic Index (TI) were calculated according to Ulbricht and Southgate (1991):AI= [C12:0 + (4 x C14:0) + C16:0]/(ω-3 + ω-6 + MUFA) TI= (C14:0 + C16:0 + C18:0)/[(0.5 x C18:1) + (0.5 x other MUFA) + (0.5 x ω-6) + (3 x ω-3) + (ω-3/ω-6)]. where: C12: 0 = lauric acid, C14: 0 = myristic acid, C16: 0 = palmitic acid, C18: 0 = stearic acid, C18: 1 = oleic acid, In the equations, C14:0 is considered to be 4 times more atherogenic than other FAs. To the C18:1, the omega 6 PUFA and to the rest of MUFA coefficients of 0.5 have been assigned because they are less anti-atherogenic than the omega 3, to which a coefficient of 3 was assigned
Statistical analysisx
All the data were analyzed using a one-way ANOVA design. Data analysis was carried out using the General Linear Model Procedures (Stat graphics- Centurion), calculated with Statistical Analysis System [33].
Results
Feeds analysis
The chemical composition of the diets fed by animals in the different breeding systems is reported in Table 1. The highest content of crude protein and the lowest NDF and ADF percentages were registered in FC while EG showed opposite results. Consequently, the diet nutritive value increased with the increase of concentrate in the diet (UFL/kg DM: 0.75 - 0.76 vs 0.78 - 0.80 vs 0.83 - 0.87, for EG, SG and FC.
Table 1:Diet Chemical Composition (% DM) and Nutritive Value (MUF/kg DM) in Exclusive Grazing (EG), Supplemented Grazing (SG) and confinement (FC) system.
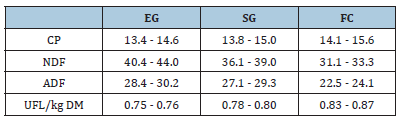
CP: Crude Protein; NDF: Neutral Detergent Fiber; ADF: Acid Detergent Fiber; UFL: net energy for lactation
Milk yield
Average milk yield was kg 7.2±1.53 vs 9.54±2.72 vs 16.20±2.12 kg, for EG, SG and FC respectively Milk chemical composition was unaffected by treatment (Table 2).
Table 2:Milk chemical composition (g/kg).
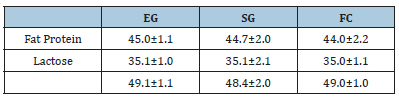
CP: crude protein; NDF: neutral detergent fiber; ADF: acid detergent fiber; UFL: net energy for lactation
Milk fatty acid profile
Table 3:Milk saturated fatty acids (SFA) profile (g/100 gfat).
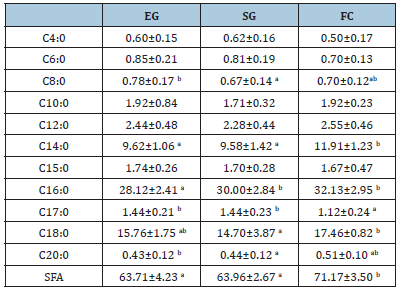
EG: Exclusive Grazing; SG: Supplemented Grazing; FC: Full Confinement
Myristic (C14:0), margaric (C17:0) and stearic (C18:0) acids, as well as total SFA, were significantly.
(P<0.05) higher in milk from FC than SG and EG. Group EG showed the lowest value of palmitic acid
(C16:0) (28.12 g/100g) statistically different (P<0.05) from both SG (30.00 g/100g) and FC (32.13g/100g) . The other SFAs were not significantly different among the breeding systems (Table 3).
Milk from SG showed the highest concentration, even if not significantly different, of unsaturated fatty acids (UFA): 35.76g/100g vs 35.09g/100g vs 34.62g/100g, for SG, EG and FC, respectively. concerning the Monounsaturated Fatty Acids (MUFA), milk from EG had the highest concentration (32.3g/100g) compared to FC (31.42 g/100g) and SG (32.27 g/100g), but, again, the differences were not significant. Similar results were found for the Polyunsaturated Fatty Acids (PUFA) concentration with no statistical difference among the breeding systems. The most representative acid in milk from all the systems was the oleic acid (C18:1) followed by linoleic acid (C18:2) and palmitoleic acid (C16:1) (Table 4). The ω6/ω3 ratio was significantly (P<0.05) different among the systems: group FC had the highest value (6.21:1) followed by SG (3.35:1) and EG (2.07:1). This result was mainly due to the different linolenic acid (C18:3) concentration among the groups. Concerning ω6 FA, a significant lower concentration (P<0.05) was found for linoleic acid (C18:2) in milk from EG compared to those from to other systems (Table 4).
Table 4:Milk Unsaturated Fatty Acids (UFA) profile (g/100g).
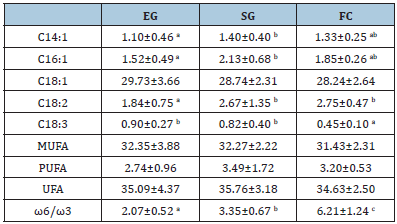
EG: Exclusive Grazing; SG: Supplemented Grazing; FC: Full Confinement
The ω6/ω3 ratio was significantly (P<0.05) different among the systems: group FC had the highest value (6.21:1) followed by SG (3.35:1) and EG (2.07:1). This result was mainly due to the different linolenic acid (C18:3) concentration among the groups. Concerning ω6 FA, a significant lower concentration (P<0.05) was found for linoleic acid (C18:2) in milk from EG compared to those from to other systems (Table 4). The ω6/ω3 ratio was significantly (P<0.05) different among the systems: group FC had the highestvalue (6.21:1) followed by SG (3.35:1) and EG (2.07:1). This result was mainly due to the different linolenic acid (C18:3) concentration among the groups. Concerning ω6 FA, a significant lower concentration (P<0.05) was found for linoleic acid (C18:2) in milk from EG compared to those from the other systems (Table 4). MUFA: monounsaturated fatty acids; PUFA: polyunsaturated fatty acids; UFA: unsaturated fatt acids; ω6/ω3: omega6/omega3 ratio.
Means with different letters indicate differences (p<0.05) among breeding systems. Both the Atherogenic (AI) and Thrombogenic (TI) indexes were higher for FC than EG and SG, but a statistical difference (P<0.05) was seen only for TI (Table 5). Means with different letters indicate differences (p<0.05) among breeding systems.
Table 5:Milk Atherogenic (AI) and Thrombogenic (TI) index.
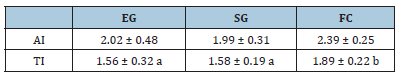
EG: Exclusive Grazing; SG: Supplemented Grazing; FC: Full Confinement
Discussion
The breeding system significantly affected milk yield which increased with the increase of concentrate in the animals’ diet. Concerning milk chemical composition, fat percentage was higher when animals had access to pasture but the differences with the full confinement system were not significant. By contrast, milk fatty acid profile was significantly healthier in the grazing than in the full confinement systems. Indeed, Park WY, et al. [21], Jensen RG [22], Chapkin RS [23], and Banskalieva V, et al. [24] discussed that fat and cholesterol have worldwide increased in human’s diet, thus becoming a serious health risk due to coronary and vascular problems. According to these authors, the consumption of saturated fatty acids, particularly lauric (C12:0) myristic (C14:0) and palmitic (16:0), are related to hypercholesterolemia due to an increase in plasma Low Density Lipoproteins (LDL) while stearic (C18:0) and oleic acid (C18:1) decrease LDL and increase High Density Lipoproteins (HDL), favoring liver formation of Very Low Density Lipoproteins (VLDL) that allows cholesterol to be transformed to gall bladder salts. Data in the present trial confirmed this low potential hypercholesterolemic effect of milk from grazing animals; in fact, milk from exclusive grazing had significantly lower contents of both myristic (C14:0) and palmitic (16:0) acids, and milk from supplemented grazing only of palmitic acid than that from full confinement system.
Despite a similar content of oleic acid (C18:1), milk from EG and SG showed 50% higher content of linolenic acid (C18:3) than that from FC group. Some studies reported modifications of milk fatty acid profile as a function of animals diet: in particular, a decrease of C16:0 and an increase of C18:0 and C18:1 content were observed in cows grazing pasture compared to cows fed a total mixed ration [25,26]. Concerning ω6 and ω3 PUFA, it has been shown that milk contents of both linoleic acid (C18:2, ω6) and linolenic acid (C18:3, ω3) are affected by the feeding system [27]. Similarly, in the present study, the highest content of linoleic acid was in milk from FC while that of linolenic acid in milk from EG. Decreasing milk ω6/ω3 ratio showed several beneficial effects for human health [5,15], particularly with values lower than 4, since higher levels could modify the beneficial effects of ω3 [14,15]. In present trial, the relationship between breeding system and milk ω6/ω3 ratio was shown: feeding animals with higher quantity of concentrates increased milk yield and ω6/ω3 ratio. Similar results were reported by Salado EE, et al. [28], in a study aimed to evaluate the effects of diets with different levels of concentrate (3.5, 7.1 and 10.5kg/day) on milk yield and quality of grazing dairy cows in early lactation. These authors registered higher milk yield and protein in groups fed 7.0 and 10.5kg/day than in group fed 3.5kg of concentrate [28- 35]. In contrast, milk fat did not differ among the groups and, even if the potential hypercholesterolemic fatty acids of milk (C12:0 to C16:0) did not change by increasing concentrate intake, linolenic acid decreased and the ω6/ω3 ratio increased in groups fed higher amounts of concentrate. Corazzin M, et al. [35] evaluated the effect of concentrate supplementation (High: 3.0kg/head/d vs. Low: 1.5 kg/ head/d) on milk fatty acid profile of Italian Simmental dairy cows grazing on alpine pasture. Low milk showed higher concentration of linolenic acid and total PUFA than High milk. Recently, Santa- Ana A, et al. [29] and Galina MA, et al. [30] compared two breeding systems for goats, full confinement or grazing: milk from animals fed on pasture showed higher PUFAs and MUFAs and lower SFAs with a significant reduction of atherogenic index, thus presumably more beneficial for human health. Musco N, et al. [31] evaluated the effects of a feeding strategy (based on the use of outdoor paddocks; forage: concentrate ratio at least 70:30; forage including at least five different herbs; and no silages) in dairy cows on milk yield and chemical composition, and blood metabolic profile, including the evaluation of oxidative stress.
These authors reported that the proposed feeding system was able to increase milk quality, mainly in terms of fats quality, without negative effects of animal health. Animals fed higher forage: concentrate diet were able to maintain body homeostasis by changing metabolism despite the low energy diet and they showed a general improvement of oxidative status, probably due to an improvement of the biological antioxidant potential. All these results showed that, even considering the differences among ruminants, management on grazing is the key component to improve omega relationship. Finally, both Milk Atherogenic (IA) and Thrombogenic Index (IT) were affected by breeding system. They take into account the potential effect of each fatty acid on human health, and they were lower for grazing than full confinement animals. Thus, milk from grazing animals should have low probability of increasing the incidence of atheroma and/or thrombus formation [32]. The possibility of producing healthier milk by grazing may be of great importance in those areas, like Mexico, still plenty of natural grasses. Also, further studies in the same areas should confirm the hypothesis that grazing may be also beneficial for animals health, thus addressing the increasing concerns about animal welfare.
Conclusion
The breeding system resulted as a fundamental aspect to determine the nutritional quality of milk, mainly related to its fatty acid profile. Despite an increase of milk yield, the full confinement system showed a worsening of healthier parameters: ω6/ω3 ratio greater than 4:1 and increase of both atherogenic and thrombogenic indexes. Therefore, in response to the consumer demand for foods with high nutritional quality, the grazing systems has to be encouraged and milk from animals with free access to pasture has to be recommended.
Ethical Statement
Experimental cows were treated following the Ley Federal de Sanidad Animal (Federal Law for Animal Health [www.diputados. gob.mx/LeyesBiblio/ref/Ifsa.htm]).
Acknowledgments
Research was supported by PAPIIT IN IT202014 and Cátedra Faculty of Higher Studies Cuautitlan, National Autonomous University of Mexico.
References
- Slots T, Butler G, Leifert C, Kristensen T, Skibsted LH, et al. (2009) Potential to differentiate milk composition by different feeding strategies. J Dairy Sci 92(5): 20157-2066.
- Tudisco R, Calabrò S, Cutrignelli MI, Moniello G, Grossi M, et al. (2012) Influence of organic systems on Stearoyl-CoA desaturase in goat milk. Small Rum Res 106: 37-42.
- Tudisco R, Grossi M, Calabrò S, Cutrignelli MI, Musco N, et al. (2014) Influence of pasture on goat milk fatty acids and Stearoyl-CoA desaturase expression in milk somatic cells. Small Rum. Res 122(1-3): 38-43
- Cabiddu A, Molie G, Decandin M (2017) Influence of the goat farming system on the vitaminic and feol composition of milk. (Influence of the goat breeding system on the vitaminic and phenolic composition of milk). Dairy Science and Technique 68(3-6): 67-68.
- Galina MA, Pineda J, Higuera-Piedrahita R, Vázquez P, Haenlein G, et al. (2019) Effect of grazying on the fatty acid composition of goat´s milk or cheese. Adv Dairy Res 7(3): 227.
- Pisoschi AM, Pop A (2015) The role of antioxidants in the chemistry of oxidative stress: A review. Eur J Med Chem 97: 55-74.
- Castel JM, Mena Y, Ruiz FA, Camuñez-Ruiz J, Sánchez-Rodriguez M (2011) Changes occurring in dairy goat production systems in less favored areas of Spain. Small Rumin Res 96(2-3): 83-92.
- Galina MA, Osnaya F, Cuchillo HM, Haenlein GFW (2007) Cheese quality from milk of grazing or indoor fed zebu cows and alpine crossbred goats. Small Rumin Res 71(1-3): 264-272.
- Claps S, Galina MA, Rubino R, Pizzillo M, Morone G, et al. (2014) Effect of grazing into the omega 3 and aromatic profile of bovine cheese. J Nutr Ecology and Food Res 2(3): 1-6.
- Galina MA, Elías A, Vázquez P, Pineda J, López B (2016) Effect of the use of fermentation promoters with or without the use of probiotics on the profile of fatty acids, amino acids and grazing cow's milk. Cuban J Agric Sci 50: 1-16.
- Simopoulos AP (2002) The importance of the ratio of omega6/omega3 essential fatty acids. Biomed Pharmaco 56(8): 365-379.
- Cavaliere G, Trinchese G, Musco N, Infascelli F, De Filippo C, et al. (2018) Milk from cows fed a diet with a high forage: Concentrate ratio improves inflammatory state, oxidative stress, and mitochondrial function in rats. J Dairy Sci 101(3): 1843-1851.
- Trinchese G, Cavaliere G, Penna E, De Filippo C, Cimmino F, et al. (2019) Milk from cow fed with high forage/concentrate ratio diet: Beneficial effect on rat skeletal muscle inflammatory state and oxidative stress through modulation of mitochondrial. Front Physiol 9: 1969.
- Colavilla G, Amadoro C, Mignona R (2014) Ratio omega6/omega3 and GPA in the noble milk in molise en r ruby. The noble milk model another way is possible, Italy, pp. 118-128.
- Gomez-Candela C, Bermejo-López L, Loria-Kohem V (2011) Importance of a balanced omega6/omega3 ratio for the maintenance of health: Nutritional recommendations. Nutr Hosp 26(2): 323-329.
- AOAC Official Methods of Analysis (2012) Association of official analytical chemists, Arlington, Texas, USA.
- Van Soest PJ, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 74(10): 3583-3598.
- INRA (1978) Feeding of ruminants, INRA, Paris, France.
- Christie WW (1992) A simple procedure of rapid transmethylation of glycerolipids and cholesteryl esters. J Lipid Res 23(7): 1072-1075.
- Chouinard PY, Corneau L, Barbano DM, Metzger LE, Bauman DE (1999) Conjugated linoleic acids alter milk fatty acid composition and inhibit milk fat secretion in dairy cows. J Nutr 129(8): 1579-1584.
- Park WY, Juárez M, Ramos M, Haenlein GFW (2016) Physico-chemical characteristics of goat and sheep milk. Small Rumin Res 68(1-2): 88-113.
- Jensen RG (2000) Fatty acids in milk and dairy products. In: Chow C (Eds.), Fatty acid in foods and their health implications. Marcel Dekker, New York, USA, pp. 109-124.
- Chapkin RS (2000) Reappraisal of the essential fatty acids. Chow C (Eds.), Fatty acid in foods and their health implications. Marcel Dekker, New York, USA, pp. 557-568.
- Banskalieva V, Sahlu T, Goetsch AL (2003) Fatty acid composition of goat muscles and fat deposits: A review. Small Rumin Res 37(3): 255-268.
- Kelly ML, Kolver ES, Bauman DE, Van Amburgh ME, Muller LD (1998) Effect of Intake of pasture on concentrations of conjugated linoleic acid in milk of lactating cows. J Dairy Sci 81(6): 1630-1636.
- Delaby L, Peyraud J, Delagarde R (2001) Effect of the level of concentrate supplementation, herbage allowance and milk yield at turn-out on the performance of dairy cows in mid lactation at grazing. Animal Sci 73(1): 171-181.
- Zlatanos S, Laskaridis K, Feist C, Sagredos A (2002) CLA content and fatty acid composition of Greek feta and hard cheeses. Food Chem 78(4): 471-477.
- Salado EE, Bretschneider G, Cuatrin A, Descalzo AM, Gagliostro GA (2017) Milk yield and composition and pasture ruminal digestion in grazing dairy cows receiving three levels of energy concentrate supplementation. Agricultural Sci 8(10): 1135-1156.
- Santa-Ana A, Bessa R, Alves S, Medeiros A, Costa R, et al. (2019) Fatty acid, volatile and sensory profiles of milk and cheese from goats raised on native samiarid pasture or in confinement. International Dairy J 91: 147-154.
- Galina MA, Ortíz-Rubio MA, Guerrero CM, Vazquez P, Pineda LJ (2016) Effect of feeding management on the nutritional composition of artisan soft cheese made with ewe’s milk. J Nutr Ecology Food Res 3: 36-42.
- Musco N, Tudisco R, Grossi M, Mastellone V, Morittu VM, et al. (2020) Effect of a high forage: Concentrate ratio on milk yield, blood parameters and oxidative status in lactating cows. Anim Prod Sci 60(12): 1531-1538.
- Ulbricht T, Southgate D(1991) Coronary heart disease: Seven dietary factors. Lancet 338(8773): 982-992.
- SAS Institute Inc (1996) Statistical analysis system, user's guide statistics, (12th edn), Version 6, Carly, North Carolina, USA, pp. 320.
- Hara A, Radin NS (1978) Lipid extraction of tissues with a low-toxicity solvent. Anal Biochem 90(1): 420-426.
- Corazzin M, Romanzin A, Sepulcri A, Pinosa M, Piasentier E, et al. (2019) Fatty acid profiles of cow’s milk and cheese as affected by mountain pasture type and concentrate supplementation. Animals (Basel) 9(2): 68.
© 2022 Miguel Ángel Galina. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






