- Submissions

Full Text
Novel Practices in Medical Study
Marine-Derived Collagen and its Hydrolysates: Health Benefits and Biological Activities
Alisson Sisa and Mauricio Mosquera*
Department of Food Science and Biotechnology (DECAB), Escuela Politécnica Nacional, Ecuador
*Corresponding author:Mauricio Mosquera, Department of Food Science and Biotechnology (DECAB), Escuela Politécnica Nacional, Quito, Ecuador
Submission: July 26, 2024;Published: August 09, 2024

NPMSVolume1 Issue4
Abstract
Collagen, an essential structural protein in the human body, is pivotal for maintaining the integrity and elasticity of skin, bones, tendons, and other connective tissues. Notably, marine-derived collagen, primarily from fish skin, scales, and bones, offers numerous health benefits and ecological advantages. This review explores the diverse biological activities of collagen hydrolysates, including Prolyl Endopeptidase (PEP) inhibitory activity, antioxidant, antimicrobial, ACE inhibitory, anticancer, hypoglycemic, antiinflammatory, immunomodulatory, joint and bone health improvement, wound healing, antiplatelet, and dermocosmetic activities. An analysis of the BIOPEP-UWM database (July 25, 2024) revealed that 71.74% of peptides contain glycine, proline, hydroxyproline, or hydroxylysine, underscoring the functional uniqueness of collagen hydrolysates. These bioactive peptides demonstrate significant potential in preventing and managing various health conditions, promoting overall well-being, and supporting sustainable production practices through marine sources.
Keywords:Marine collagen; Collagen hydrolysates; Bioactive peptides; Health benefits; Biological activities; Sustainable production
Abbreviations:ACE: Angiotensin-Converting Enzyme; DPP-IV: Dipeptidyl Peptidase IV; HUVECs: Human Umbilical Vein Endothelial Cells; IC50: Half Maximal Inhibitory Concentration; iNOS: Inducible Nitric Oxide Synthase; LHN: Low-Molecular-Weight and High-Dose Nanoemulsion; LMWCP: Low-Molecular- Weight Fish Collagen Peptides; MSCP: Milkfish Scale Collagen-Derived Peptides; NF-κB: Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells; NO: Nitric Oxide; PEP: Prolyl Endopeptidase; PGE2: Prostaglandin E2; TLR: Toll-Like Receptor
Introduction
Collagen is an essential structural protein in the human body, present in the skin, bones, tendons, and other connective tissues. It constitutes approximately 30% of the body’s total proteins and is crucial for maintaining the integrity and elasticity of these tissues. Notably, collagen, particularly type I, is a common protein in nearly all vertebrates, making it one of the most abundant and conserved proteins in the animal kingdom. This type of collagen forms large eosinophilic fibers and is the main structural component of various tissues such as skin, bones, tendons, and ligaments in humans and other species. The structural similarity of type I collagen among different species allows the human body to efficiently recognize and utilize collagen-derived peptides when consuming animal-derived collagen. Throughout life, collagen production decreases, leading to various health issues such as loss of bone density, reduced skin elasticity, and joint problems. This deterioration can be countered by supplementing with collagen and its derivatives, such as collagen hydrolysates. Collagen hydrolysates are obtained by breaking down collagen into smaller peptides, which are easily absorbed by the body.
These bioactive peptides have demonstrated multiple health benefits, including the ability to inhibit Prolyl Endopeptidase (PEP), potentially preventing neurodegenerative damage; antioxidant activity, which neutralizes free radicals and reduces oxidative stress; and antimicrobial activity, which can disrupt the cell membrane of bacteria and other microorganisms. They have also been shown to inhibit Angiotensin-Converting Enzyme (ACE), crucial for blood pressure control, and possess vasodilatory properties, improving blood flow and reducing blood pressure. In addition, these peptides have exhibited anticancer activity by inhibit- ing the growth of cancer cells, hypoglycemic activity by inhibiting Dipeptidyl Peptidase IV (DPP-IV), aiding in blood sugar control, and anti-inflammatory activity, reducing inflammation and alleviating pain. Other activities include immune response modulation, joint and bone health improvement by stimulating collagen synthesis in cartilage and bones, wound healing acceleration, prevention of blood clot formation, and dermo cosmetic benefits, enhancing skin elasticity and appearance, among others. Research into alternative collagen sources, such as marine sources, highlights the importance of diversifying collagen sources to ensure sustainable and efficient production. Marine collagen, derived mainly from the skin, scales, and bones of fish, offers several advantages over traditional sources. This type of collagen is not only easier to digest and absorb due to its smaller molecular structure but also has a lower environmental impact, utilizing by-products of the fishing industry, thereby reducing waste.
Discussion
Prolyl Endopeptidase (PEP) inhibitory activity
The inhibition of Prolyl Endopeptidase (PEP) is a significant activity of collagen-derived peptides, as this enzyme is involved in neurodegenerative processes. PEP is responsible for the degradation of proteins that regulate brain function, and its inhibition can help prevent neurodegenerative diseases such as Alzheimer’s. Collagen hydrolysates have shown significant PEP inhibitory activity, suggesting their potential in protecting against cognitive decline. Research has demonstrated that gelatin polypeptides from barbel skin (Barbus callensis) are a good source of natural PEP inhibitors, with the highest PEP-inhibiting activity observed having an IC50 value of 0.91mg/ml when hydrolyzed with trypsin [1]. Additionally, enzymatic hydrolysis of unmarketable prawn proteins using alkaline protease produced hydrolysates with significant PEP-inhibitory activity, achieving a maximum inhibitory value of 40.9% after 10 minutes of hydrolysis [2]. These hydrolysates exhibit varied molecular weights, and the degree of hydrolysis influences their inhibitory activity, emphasizing the importance of peptide sequence in determining their effectiveness. This property is particularly important in the context of aging and the prevention of neurodegenerative diseases, supporting the potential of marine-derived collagen- derived peptides in neuroprotection.
Antioxidant activity
Collagen hydrolysates have also demonstrated antioxidant properties, crucial for neutralizing free radicals and reducing oxidative stress. This stress is implicated in aging and various chronic diseases, such as cardiovascular diseases and cancer. Collagen-derived antioxidant peptides can capture and neutralize free radicals, preventing oxidative damage to cells and tissues. This antioxidant capacity has been observed in studies with peptides obtained from fish scales and squid tunics. For instance, peptides derived from the hydrolysis of jumbo squid (Dosidicus gigas) tunics using Alcalase and Esperase showed a high radical scavenging ability, with ABTS values around 43mg VCEAC/g sample [3]. Similarly, peptides from gilthead seabream (Sparus aurata) scales exhibited significant antioxidant activity, with an IC50 value of 27.7±0.5 mg VCEAC/g protein for ABTS radical scavenging [4]. The antioxidant activity of these peptides is strongly related to their molecular weight, with lower molecular weight peptides (<1kDa) showing the highest activity. Studies have shown that supplementation with these peptides can increase the body’s antioxidant capacity, providing an effective defense against free radical damage and promoting healthier skin, better cardiovascular function, and increased longevity [5].
Antimicrobial activity
Collagen hydrolysates have also demonstrated significant antimicrobial properties. These peptides can disrupt the cell membrane of bacteria and other microorganisms, providing potential use as natural antimicrobial agents. Research has shown that collagen-derived peptides derived from gilthead seabream scales and squid tunics can inhibit the growth of various bacterial strains, such as Bacillus cereus and Bacillus coagulans [3]. The antimicrobial activity of these peptides is associated with their content of hydrophobic amino acids, which facilitate interaction with the cytoplasmic membranes of microorganisms. This property is particularly valuable in the formulation of food and cosmetic products, providing a natural and safe alternative to synthetic preservatives.
Angiotensin-Converting Enzyme (ACE) inhibitory activity
The inhibition of Angiotensin-Converting Enzyme (ACE) is crucial in blood pressure control, as this enzyme catalyzes the conversion of angiotensin I to angiotensin II, a potent vasoconstrictor. ACE inhibitory peptides derived from marine collagen, such as those obtained from gilthead seabream (Sparus aurata) scales, can offer a natural alternative to synthetic inhibitors, reducing the risk of associated side effects. Studies have demonstrated that collagen- derived peptides with a lower molecular weight (<3kDa) show high ACE inhibitory activity, indicating their potential in managing hypertension. For example, hydrolysates from gilthead seabream scales obtained using Esperase, exhibited an IC50 value of 59.9mg/ mL for ACE inhibition [6]. Similarly, collagen-derived peptides derived from the skin of Takifugu bimaculatus showed ACE inhibitory activity with an IC50 value of 0.54mg/mL. The peptide FNLRMQ from this source demonstrated significant protective effects against angiotensin II-induced injury in Human Umbilical Vein Endothelial Cells (HUVECs), indicating its potential inantihypertensive therapy. This peptide was effective in alleviating the viability and facilitating apoptosis of Ang-II-induced HUVECs by regulating Nrf2/HO-1 and PI3K/Akt/eNOS signaling pathways [7]. These findings underscore the effectiveness of marine-derived collagen-derived peptides in ACE inhibition and their potential application in hypertension management.
Anticancer activity
The anticancer activity of collagen-derived peptides has been the subject of various studies. These peptides can inhibit the growth of cancer cells by inducing apoptosis and inhibiting cell proliferation. Research has shown that marine collagen-derived peptides, such as those obtained from giant squid (Dosidicus gigas) tunics, have significant effects in inhibiting the growth of various cancer cell lines. For instance, hydrolysates prepared from squid gelatin using enzymes like Esperase and Alcalase showed notable cytotoxic and antiproliferative activities. The Esperase hydrolysate exhibited the highest cytotoxic effect on both MCF-7 (breast cancer) and U87 (glioblastoma) cell lines, with viability inhibition rates of 96.6±0.5% and 91.2± 2.7%, respectively, after 72 hours at a concentration of 1mg/mL. The IC50 values for the Esperase hydrolysate were 0.13mg/mL for MCF-7 cells and 0.10mg/mL for U87 cells, indicating strong efficacy. The Alcalase hydrolysate also showed significant cytotoxic activity, with inhibition rates of 67.8±5.6% for MCF-7 cells and 83.9±4.8% for U87 cells [8]. Similarly, collagen-derived peptides derived from rainbow trout (Oncorhynchus mykiss) skin hydrolyzed with Alcalase and Flavourzyme demonstrated significant anticancer activity. The hydrolysates were fractionated by molecular weight, and the <3kDa fraction exhibited the highest Inhibitory Concentration (IC50) against HCT-116 colon cancer cells. The IC50 values were 727.4μg/mL for the Flavourzyme hydrolysate and 249.5μg/mL for the Alcalase hydrolysate, highlighting their potential as natural anticancer agents [9]. These findings suggest that collagen-derived peptides, particularly those hydrolyzed with specific enzymes such as Esperase, Alcalase, and Flavourzyme, could be promising sources of natural anticancer agents, providing an alternative to conventional chemotherapy with potentially fewer side effects.
Hypoglycemic activity
The hypoglycemic activity of collagen-derived peptides has been demonstrated through their ability to inhibit Dipeptidyl Peptidase IV (DPP-IV), an enzyme that degrades incretins, hormones that stimulate insulin release. By inhibiting DPP-IV, collagen-derived peptides can help control blood sugar levels, which is particularly beneficial for people with type 2 diabetes. Research has shown that peptides obtained from marine sources, such as gilthead seabream (Sparus aurata) scales, have significant DPP-IV inhibitory activity. For example, peptides derived from gilthead seabream scales hydrolyzed with Alcalase demonstrated an IC50 value of 0.9mg/ mL, indicating their strong potential in blood glucose regulation [1]. Similarly, collagen-derived peptides from tilapia skin gelatin hydrolysate produced using ginger protease also exhibited potent DPP-IV inhibitory effects, with the most active peptide, GPXGPPGPGP, showing an IC50 value of 1012.3μM and demonstrating rapid binding and dissociation kinetics with DPP-IV [10]. These findings suggest that marine-derived collagen-derived peptides could be a promising source of natural hypoglycemic agents, offering a complementary strategy for managing type 2 diabetes.
Anti-inflammatory activity
Collagen-derived peptides have also demonstrated anti-inflammatory properties, reducing inflammation and alleviating pain. These properties are due to the ability of peptides to inhibit the production of inflammatory mediators and modulate the immune response. Studies have shown that collagen hydrolysates can significantly reduce inflammation in experimental models, suggesting their potential in treating chronic inflammatory conditions such as arthritis. For example, marine collagen-derived peptides from milkfish (Chanos chanos) scales have been shown to reduce lipoxygenase activity and Nitric Oxide (NO) production, both of which are key mediators in the inflammatory process. The Milkfish Scale Collagen- Derived Peptides (MSCP) exhibited dose-dependent anti-inflammatory activity, with significant inhibition of lipoxygenase and NO production at concentrations of 0.5 and 1mg/mL, showing an IC50 value of 475.9μg/mL for lipoxygenase inhibition and 742.5μg/ mL for NO production inhibition [11]. Additionally, peptides from ethanol-soluble hydrolysates of sturgeon (Acipenser schrenckii) cartilage also exhibited strong anti-inflammatory effects. These hydrolysates were able to significantly reduce the production of pro-inflammatory cytokines such as IL-6 and increase the levels of anti-inflammatory cytokines like IL-10 in LPS-induced RAW264.7 macrophages [12]. The anti-inflammatory activity of these collagen- derived peptides is linked to their ability to modulate key signaling pathways involved in inflammation, highlighting their potential as natural anti-inflammatory agents.
Immunomodulatory activity
Collagen-derived peptides can modulate the immune response, enhancing the body’s defense against infections and diseases. This immunomodulatory activity has been observed in studies where collagen hydrolysates increased the production of immune cells and improved overall immune response. For example, low molecular- weight peptides from Nibea japonica have demonstrated significant immunomodulatory effects in RAW264.7 cells. These peptides promoted cell proliferation, increased phagocytic activity, and elevated the secretion of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β. Additionally, these peptides enhanced the synthesis of Nitric Oxide (NO) by upregulating Inducible Nitric Oxide Synthase (iNOS) protein levels, further boosting the immune response. The activation of key signaling pathways, such as the NF- κB pathway, which regulates the expression of genes involved in the immune response and inflammation, was also observed [13]. Similarly, peptides isolated from the protein hydrolysate of yellowfin tuna (Thunnus albacares) trimmings demonstrated significant immunomodulatory effects. The fraction T1 of the hydrolysate enhanced phagocytosis, promoted the secretion of Nitric Oxide (NO), IL-1β, IL-6, and TNF-α, and activated the NF-κB signaling pathway in RAW264.7 cells. These peptides also showed a strong binding affinity to Toll-Like Receptors (TLR2 and TLR4), indicating their role in immune system modulation [14]. These findings suggest that marine-derived collagen peptides could be a promising source of natural immunomodulatory agents, enhancing the immune response through multiple pathways and mechanisms.
Joint and bone health improvement
Collagen is an essential component of bones and joints, and collagen hydrolysates can stimulate collagen synthesis in cartilage, improving joint health and alleviating symptoms of conditions such as osteoarthritis. Studies have shown that supplementation with collagen- derived peptides can improve bone mineral density and reduce joint pain, providing significant benefits for people with joint problems. For example, research with marine-derived collagen hy drolysates has demonstrated an improvement in bone density and a reduction in pain in osteoarthritis models. Specifically, Low-Molecular- Weight Fish Collagen Peptides (LMWCP) from tilapia (Oreochromis genus) gelatin have shown promising results [15]. These peptides were found to enhance osteoblastic activity and promote the synthesis of type I collagen, crucial for bone formation and repair. LMWCP significantly increased the mRNA expression levels of anabolic factors such as aggrecan, collagen type I, collagen type II, TIMP-1, and TIMP-3, while decreasing the expression of catabolic factors like MMP-3 and MMP-13 in H2O2-treated chondrocytes. Additionally, LMWCP treatment significantly ameliorated inflammation by reducing levels of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6), Prostaglandin E2 (PGE2), and Nitric Oxide (NO) in LPS-treated chondrocytes. Furthermore, research has highlighted the role of collagen peptides in bone and cartilage regeneration. Peptides derived from fish scale collagen have been shown to stimulate osteoblast differentiation, enhancing bone mineralization and osteogenic gene expression. These bioactive peptides mimic the signaling domains of larger proteins, promoting cell adhesion, proliferation, and differentiation, essential for bone and cartilage repair [16]. These findings suggest that collagen peptides from marine sources, such as tilapia, can be effective in supporting joint and bone health through multiple mechanisms, including anti-inflammatory effects, enhanced collagen synthesis, and the suppression of cartilage degradation.
Wound healing activity
Collagen-derived peptides have also been shown to improve wound healing, accelerating the skin repair process. This wound healing activity is due to the ability of peptides to stimulate collagen synthesis and other extracellular matrix components at the wound site. Studies have shown that collagen hydrolysates can significantly accelerate wound healing in animal models, suggesting their potential in treating wounds and ulcers. For example, Marine Collagen Peptides (MCPs) from the skin of Nile tilapia (Oreochromis niloticus) were found to be composed mainly of polypeptides with molecular weights less than 5kDa, which facilitated better absorption and enhanced water solubility. These MCPs significantly improved the scratch closure rate in vitro with concentrations of 50.0μg/mL almost completely closing the scratch wound within 24 hours. In vivo experiments with rabbits demonstrated that MCPs significantly accelerated the wound healing process of deep partial- thickness scald wounds, showing a notable increase in the healing rate and quality of the repaired tissue. Additionally, the MCPs were observed to enhance the migration of keratinocytes, which is crucial for re-epithelialization during wound healing [17].
Similarly, low-molecular-weight collagen peptides from sturgeon fish (Acipenser baerii) skin significantly increased wound healing in diabetic mice. These peptides accelerated the wound healing process by promoting collagen synthesis, reducing inflammation, and improving the overall quality of the healed tissue. Specifically, the Low-Molecular-Weight and High-Dose Nanoemulsion (LHN) group showed the most pronounced effect in maintaining the largest wound-healing area (95.53%) over a 15-day period in diabetic mice, highlighting the potential of these peptides as effective agents for wound healing applications [18]. These findings indicate that collagen peptides from marine sources, such as Nile tilapia and sturgeon fish, can support and accelerate the wound healing process through multiple mechanisms, including improved cell migration, collagen synthesis, and enhanced tissue regeneration.
Antiplatelet activity
Collagen-derived peptides have demonstrated antiplatelet activity, which can prevent the formation of blood clots and reduce the risk of thrombosis. This activity has been observed in studies where collagen hydrolysates inhibited platelet aggregation, a key step in clot formation. Research has shown that marine-derived collagen peptides can significantly reduce platelet aggregation in experimental models, suggesting their potential in preventing cardiovascular diseases. For instance, in a study, Alcalase protease and Protamex protease were used to hydrolyze the skin collagen of silver carp (Hypophthalmichthys molitrix) to obtain different hydrolysates (AH and PH). The antiplatelet activity of these hydrolysates was evaluated in models induced by collagen, thrombin, and ADP. The results indicated that AH significantly inhibited platelet aggregation caused by all three inducers, while PH showed significant antiplatelet activity only in the ADP-induced platelet aggregation model. Further separation and identification of antiplatelet peptides from these hydrolysates revealed that several peptides had high inhibitory effects on ADP-induced platelet aggregation.
The peptide OGSA exhibited the highest antiplatelet activity, with an IC50 of 0.63mM. In vivo studies using the FeCl3-induced arterial thrombosis model in rats demonstrated that OGSA could inhibit thrombus formation significantly at doses of 200 and 300μM/ kg body weight, comparable to the positive control, clopidogrel [19]. In another study, focused on the skin collagen of Atlantic salmon (Salmo salar) hydrolyzed with Alcalase and Protamex® also revealed significant antiplatelet activity. The peptides Hyp-Gly- Glu-Phe-Gly (OGEFG) and Asp-Glu-Gly-Pro (DEGP) from this study showed potent activity against ADP-induced platelet aggregation with IC50 values of 277.17μM and 290.00μM, respectively. In vivo experiments demonstrated that these peptides could significantly inhibit thrombus formation at doses of 200 and 300μmol/kg body weight without prolonging bleeding time or causing an immune response [20]. These findings collectively suggest that collagen peptides can effectively inhibit platelet aggregation, thereby potentially reducing the risk of thrombosis and cardiovascular diseases.
Dermocosmetic activity
Collagen-derived peptides offer notable dermocosmetic benefits by enhancing skin elasticity and appearance through the stimulation of collagen synthesis and other extracellular matrix components. Clinical trials have consistently shown that these peptides improve skin hydration, elasticity, and firmness, effectively reducing signs of aging. Notably, collagen extracted from the skins of Atlantic codfish (Gadus morhua) and Atlantic salmon (Salmo salar) has yielded high-purity type I collagen with excellent moisturizing capabilities and non-irritant properties suitable for dermal appli cations [21]. Specifically, salmon collagen exhibits superior structural preservation, as evidenced by its triple helix structure, and demonstrates significant hydration retention capabilities. These marine-derived collagens have shown promising results in enhancing skin moisturization and preventing dehydration without causing skin irritation, making them valuable ingredients for cosmetic formulations. This comprehensive understanding underscores the dermocosmetic potential of collagen and its derivatives, supporting their use in enhancing skin health and appearance.
Analysis
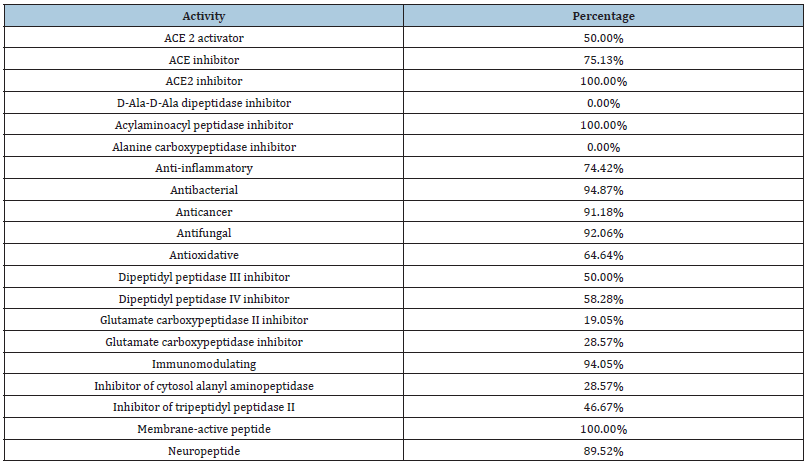
Collagen is a protein characterized by a helix formed by three chains of approximately 1000 amino acids and a unique amino acid composition, which confers its special structural properties. The most characteristic and abundant amino acids in collagen include glycine, which represents about one-third of the total collagen composition; proline, along with hydroxyproline, which helps stabilize the helical structure of collagen through hydrogen bonds; and hydroxylysine, which is produced from lysine through hydroxylation and participates in the formation of cross-links that provide additional strength and stability to the collagen matrix. These amino acids make collagen hydrolysates functionally unique in some cases for both animal and human organisms. To better understand this statement, an analysis of the BIOPEP-UWM database was conducted (July 25, 2024), focusing on the previously described biological activities by comparing 4041 peptide sequences recorded in this database. This analysis revealed that 71.74% of the peptides in the database contain glycine, proline, hydroxyproline, or hydroxylysine. The analyzed activities are shown in (Table 1). While this analysis explores the general benefits of collagen regardless of its animal origin, it is important to highlight that marine-derived collagen contributes to these benefits by offering an environmentally sustainable alternative and providing a viable option for individuals who prefer not to use terrestrial animal sources for various reasons.
Table 1:Percentage of bioactive marine-derived collagen peptides containing glycine, proline, hydroxyproline, or hydroxylysine.
Conclusion
This review elucidates the significant health benefits and biological activities of marine-derived collagen and its hydrolysates, reinforcing the theory that collagen’s unique amino acid composition confers its functional properties. The analysis of the BIOPEP- UWM database supports the assertion that the presence of glycine, proline, hydroxyproline, and hydroxylysine in collagen peptides is integral to their bioactivity. The findings demonstrate that these peptides exhibit a wide range of beneficial effects, including antioxidant, antimicrobial, anti-inflammatory, and wound healing activities, as well as the potential to manage hypertension, diabetes, and cancer. These results validate the potential of marine collagen as a valuable resource for therapeutic applications, emphasizing its role in promoting human health and offering sustainable production alternatives. The study’s findings align with the theory that the structural properties of collagen peptides are crucial for their di verse biological activities, providing a robust framework for future research and practical applications in health and wellness.
Acknowledgement
This study was supported by Project PIGR 23-10 entitled “Utilization of Agricultural By-Products in the Production of Proteases for the Obtaining of Peptide Hydrolysates with Biological Activities through Microorganisms Isolated from the Three Regions of Continental Ecuador,” conducted at the Department of Food Science and Biotechnology (EPN-DECAB).
References
- Sila A, Alvarez OM, Haddar A, Guillén MCG, Nasri M, et al. (2015) Recovery, viscoelastic and functional properties of barbel skin gelatine: Investigation of anti-DPP-IV and anti-prolyl endopeptidase activities of generated gelatine polypeptides. Food Chem 168: 478-486.
- Bougatef H, Sila A, Bougatef A, Alvarez OM (2024) Protein hydrolysis as a way to valorise squid-processing byproducts: Obtaining and identification of ACE, DPP-IV and PEP inhibitory peptides. Mar Drugs 22(4): 156.
- Mosquera M, Giménez B, Ramos S, Caballero MEMEL, Guillén MDCMG, et al. (2016) Antioxidant, ACE-inhibitory, and antimicrobial activities of peptide fractions obtained from dried giant squid tunics. Journal of Aquatic Food Product Technology 25(3): 444-455.
- Mosquera M, Giménez B, Silva IMD, Boelter JF, Montero P, et al (2014) Nanoencapsulation of an active peptidic fraction from sea bream scales collagen. Food Chem 156: 144-150.
- Wichansawakun S, Buttar HS (2019) Antioxidant diets and functional foods promote healthy aging and longevity through diverse mechanisms of action. The Role of Functional Food Security in Global Health 1: 541-563.
- Akagündüz Y, Mosquera M, Giménez B, Alemán A, Montero P, et al. (2014) Sea bream bones and scales as a source of gelatin and ACE inhibitory peptides. LWT-Food Science and Technology 55(2): 579-585.
- Cai S, Pan N, Xu M, Su Y, Qiao K, et al. (2021) ACE inhibitory peptide from skin collagen hydrolysate of Takifugu bimaculatus as potential for protecting HUVECs injury. Mar Drugs 19(12): 655.
- Alemán A, Santín EP, Juchereau SB, Arnaudin I, Guillén MCG, et al. (2011) Squid gelatin hydrolysates with antihypertensive, anticancer and antioxidant activity. Food Research International. 44(4): 1044-1051.
- Yaghoubzadeh Z, Ghadikolaii FP, Kaboosi H, Safari R, Fattahi E (2020) Antioxidant activity and anticancer effect of bioactive peptides from rainbow trout (Oncorhynchus mykiss) skin hydrolysate. Int J Pept Res Ther 26(1): 625-632.
- Liu W, Wang X, Yang W, Li X, Qi D, et al. (2022) Identification, screening, and comprehensive evaluation of novel DPP-IV inhibitory peptides from the tilapia skin gelatin hydrolysate produced using ginger protease. Biomolecules12(12): 1866.
- Chen YP, Liang CH, Wu HT, Pang HY, Chen C, et al. (2018) Antioxidant and anti-inflammatory capacities of collagen peptides from milkfish (Chanos chanos) scales. J Food Sci Technol 55(6): 2310-2317.
- Yuan L, Chu Q, Wu X, Yang B, Zhang W, et al. (2021) Anti-inflammatory and antioxidant activity of peptides from ethanol-soluble hydrolysates of sturgeon (Acipenser schrenckii) cartilage. Front Nutr 8: 689648.
- Zhang Z, Hu X, Lin L, Ding G, Yu F (2019) Immunomodulatory activity of low molecular-weight peptides from nibea japonica in RAW264.7 Cells via NF-κB Pathway. Mar Drugs 17(7): 404.
- Cai B, Chen H, Wan P, Luo L, Ye Z, et al. (2022) Isolation and identification of immunomodulatory peptides from the protein hydrolysate of tuna trimmings (Thunnas albacares). LWT 164: 113614.
- Cho W, Park J, Kim J, Lee M, Park SJ, et al. (2023) Low-molecular-weight fish collagen peptide (valine-glycine-proline-hydroxyproline-glycine-proline-alanine-glycine) prevents osteoarthritis symptoms in chondrocytes and monoiodoacetate-injected rats. Mar Drugs 21(12): 608.
- Kapat K, Kumbhakarn S, Sable R, Gondane P, Takle S, et al. (2024) Peptide-based biomaterials for bone and cartilage regeneration. Biomedicines 12(2): 313.
- Hu Z, Yang P, Zhou C, Li S, Hong P (2017) Marine collagen peptides from the skin of nile tilapia (Oreochromis niloticus): Characterization and wound healing evaluation. Mar Drugs 15(4): 102.
- Hou NT, Chen BH (2023) Preparation of nanoemulsions with low-molecular-weight collagen peptides from sturgeon fish skin and evaluation of anti-diabetic and wound-healing effects in mice. Pharmaceutics 15(9): 2304.
- Tian Q, Li SM, Li B (2021) The pro-gly or hyp-gly containing peptides from absorbates of fish skin collagen hydrolysates inhibit platelet aggregation and target P2Y12 receptor by molecular docking. Foods 10(7): 1553.
- Yang Y, Wang B, Tian Q, Li B (2020) Purification and characterization of novel collagen peptides against platelet aggregation and thrombosis from salmo salar. ACS Omega 5(32): 19995-20003.
- Alves AL, Marques ALP, Martins E, Silva TH, Reis RL (2017) Cosmetic potential of marine fish skin collagen. Cosmetics 4(4): 39.
© 2024. Mauricio Mosquera. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






