- Submissions

Full Text
Novel Approaches in Cancer Study
Current Insights on the Prevalence, Clinical Diagnosis and Management of Pancreatic Cancer: A Comprehensive Review
Moses Adondua Abah1*, Nathan Rimamsanati Yohanna1, Esther Edesiri Ajoku2, Micheal Abimbola Oladosu3, Macdonald Uchenna Eke4, Naomi Ngozichukwuka Ajoniloju5,6, Njideka Obioma Nwanne4, Onyinye Ifeoma Ikedionwu7, Odusanya Kikunlore Elijah8, Jennifer Nnaemeka9, Ekpenyong Utomobong Sunday10 and Okwah Micah Nnabuko11
1Department of Biochemistry, Faculty of Biosciences, Federal University Wukari, Nigeria
2Healthcare Management and Administration, University of New Orleans, USA
3Department of Biochemistry, Faculty of Basic Medical Sciences, University of Lagos, Nigeria
4Department of Medicine, College of Medicine, Imo State University, Nigeria
5Department of Pharmacology and Therapeutics, Faculty of Basic Clinical Sciences, Bowen University, Nigeria
6Department of Pharmacology and Therapeutics, Faculty of Basic Medical Sciences, University of Ibadan, Nigeria
7Department of Internal Medicine and Surgery, Faculty of Clinical Sciences, College of Medicine, University of Lagos, Nigeria
8Department of hematology and blood transfusion, Olabisi Onabanjo University Teaching hospital, Nigeria
9Department of Chemistry, Faculty of Arts and Sciences, Prairie View A&M University, USA
10Department of Internal Medicine, College of Medicine, University of Uyo Teaching Hospital, Nigeria
11Department of Chronic Disease Epidemiology, Yale University, United States
*Corresponding author:Moses Adondua Abah, Department of Biochemistry, Faculty of Biosciences, Federal University Wukari, Wukari, Taraba State, Nigeria
Submission: September 09, 2025;Published: November 13, 2025

ISSN:2637-773XVolume8 Issue 2
Abstract
Pancreatic cancer is a highly aggressive malignancy with a poor prognosis, accounting for a significant number of cancer-related deaths worldwide. Despite its rising incidence, the five-year relative survival rate remains low at 13%, with adenocarcinoma having a survival rate of just 8%. Early detection and effective management of pancreatic cancer are crucial to improving patient outcomes. Recent advancements in diagnostic technologies, including liquid biopsies, molecular imaging, and artificial intelligence-driven approaches, have shown promise in improving early detection rates. Liquid biopsies, in particular, offer a non-invasive means of detecting circulating cells, DNA and exosomes in bodily fluids. Meanwhile, advancements in genomic science have led to the development of targeted therapies, such as KRAS inhibitors, and immunotherapies, including checkpoint inhibitors and cancer vaccines. The management of pancreatic cancer requires a multidisciplinary approach, incorporating surgery, chemotherapy and radiation therapy. Novel therapeutic strategies, including precision medicine approaches and nanoparticle-based drug delivery systems, are being explored to enhance treatment efficacy. Clinical trials are ongoing to evaluate the safety and efficacy of these emerging treatments. This review aimed at providing current insights into the prevalence, clinical diagnosis, and management of pancreatic cancer. The complexities of pancreatic cancer diagnosis and treatment, emphasizing the need for continued research and clinical trials to improve patient outcomes were also discussed in this review.
Keywords:Pancreatic cancer; Clinical diagnosis; Targeted therapies; KRAS inhibitors; Precision medicine
Introduction
Pancreatic cancer is a malignant disease that begins when abnormal cells in the pancreas grow uncontrollably and form a tumour. The pancreas is a gland located deep in the abdomen that produces enzymes for digestion and hormones for blood sugar regulation. Functionally, it has both exocrine components responsible for secreting digestive juices into the small intestine and endocrine components responsible for producing hormones such as insulin and glucagon. Most pancreatic cancers originate in the exocrine cells, which produce digestive juices and are classified as pancreatic ductal adenocarcinoma (PDAC), the most common and aggressive subtype [1,2]. Pancreatic cancer is one of the most aggressive and lethal malignancies globally, ranking as the seventh leading cause of cancer-related deaths. Despite accounting for only 3% of all cancers, it contributes disproportionately to cancer mortality due to its insidious onset, rapid progression and limited therapeutic options. According to recent global cancer statistics, the incidence of pancreatic cancer is rising, with over 495,000 new cases and 466,000 deaths reported worldwide in 2020 alone [3]. In many regions, including sub-Saharan Africa, underreporting and limited access to diagnostic tools may obscure the true burden of the disease, making accurate prevalence data a persistent challenge. The most common histological subtype, PDAC, comprises over 90% of cases and is characterized by its resistance to conventional therapies and early metastatic potential (American Cancer Society, 2024). Risk factors include age, smoking, chronic pancreatitis, diabetes mellitus, obesity and certain genetic syndromes. The disease often remains asymptomatic until it reaches an advanced stage, contributing to its poor prognosis. Symptoms such as jaundice, weight loss, abdominal pain, and anorexia typically emerge late, by which time curative treatment is rarely feasible. As a result, the five-year survival rate remains below 10%, and median survival for metastatic disease is less than six months.
Early and accurate diagnosis is critical to improving outcomes, yet remains challenging due to the pancreas’s deep anatomical location and the nonspecific nature of early symptoms. Imaging modalities play a central role in the diagnostic process. Computed Tomography (CT), particularly multiphasic contrast-enhanced scans, is the first-line tool for detecting pancreatic masses and assessing resectability. Magnetic Resonance Imaging (MRI) offers superior soft tissue contrast and is especially useful for characterizing indeterminate lesions and evaluating ductal anatomy via MRCP. Positron Emission Tomography–Computed Tomography (PET-CT) provides metabolic insights and is valuable for staging and detecting distant metastases. Endoscopic Ultrasound (EUS), with its high-resolution imaging and ability to guide fine-needle aspiration (FNA), is indispensable for tissue diagnosis and staging [4]. Together, these modalities form a comprehensive diagnostic framework that informs clinical decision-making.
Management of pancreatic cancer depends on the stage at diagnosis and the patient’s overall health. Surgical resection remains the only potentially curative option, typically reserved for localized tumours without major vascular involvement. The Whipple procedure (pancreaticoduodenectomy) is the most common surgical approach for tumours in the pancreatic head. However, only 15-20% of patients are eligible for surgery at the time of diagnosis. For unresectable or metastatic disease, systemic chemotherapy is the mainstay of treatment. Regimens such as FOLFIRINOX or gemcitabine with nab-paclitaxel have shown modest survival benefits. Radiation therapy may be used in select cases for local control or palliation. Emerging therapies, including immunotherapy, targeted agents, and personalized medicine approaches, are under investigation but have yet to yield transformative results (Tempero et al., 2023). Supportive care is also a vital component of management, addressing pain, nutritional challenges, and psychological distress. Multidisciplinary care involving oncologists, surgeons, radiologists, gastroenterologists, and palliative care specialists is essential for optimizing patient outcomes. In resource-limited settings, barriers such as delayed diagnosis, lack of specialized care and limited access to advanced imaging and treatment modalities further complicate management and contribute to poor survival rates. This study aimed at providing current insights into the prevalence, clinical diagnosis, and management of pancreatic cancer. By synthesizing recent data and highlighting advances in diagnostic imaging and therapeutic strategies, we seek to underscore the importance of early detection, multidisciplinary care, and ongoing research in improving outcomes for this devastating disease.
Epidemiology and Risk Factors of Pancreatic Cancer
As documented by world cancer research, pancreatic cancer accounts for over 510,000 new cases and approximately 467,000 deaths annually, making it the seventh leading cause of cancerrelated mortality [5]. Table 1 summarizes the age-standardized incidence rate (ASIR) that has steadily increased over the past three decades, rising from 5.47 per 100,000 in 1990 to 5.96 per 100,000 in 2021 [6]. This upward trend is particularly pronounced in highincome countries such as Japan, the United States and Germany, where lifestyle and aging populations contribute significantly to disease burden. The five-year survival rate for pancreatic cancer remains dismally low, hovering around 10-13% [7]. This is largely due to the asymptomatic nature of early-stage disease and the lack of effective screening tools for the general population. Most patients are diagnosed at stage III or IV, when curative surgical options are no longer viable. Consequently, mortality rates closely mirror incidence rates, underscoring the urgent need for improved early detection strategies.
Table 1:Incidence and mortality rate as a result of pancreatic cancer in some nations across the globe (country-specific data).
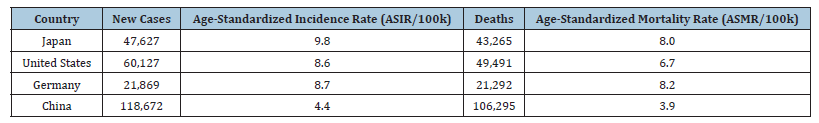
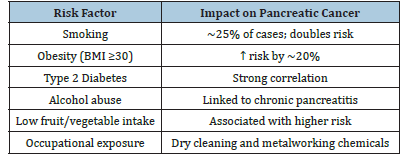
Table 2:Summary of lifestyle and environmental risk factors of pancreatic cancer.
Source: Zeng et al. [6].
Pancreatic cancer is multifactorial, with both genetic predispositions and environmental exposures playing critical roles. Approximately 10% of cases are attributed to hereditary factors, including mutations in genes such as BRCA1, BRCA2, PALB2, ATM, and CDKN2A [8]. Individuals with these mutations often have a significantly elevated lifetime risk, particularly when combined with a family history of pancreatic or related cancers. As summarized in Table 2 lifestyle factors also contribute substantially to disease risk. Cigarette smoking is one of the most well-established modifiable risk factors, accounting for nearly 25% of pancreatic cancer cases and doubling the risk compared to non-smokers [9]. Obesity, particularly with a body mass index (BMI) over 30, has been linked to a 20% increase in risk, while chronic alcohol consumption and type 2 diabetes are associated with both direct and indirect pathways of carcinogenesis [6]. Diets low in fruits and vegetables and high in processed meats may further exacerbate risk, though evidence remains mixed. Environmental exposures, such as prolonged contact with industrial chemicals used in dry cleaning and metalworking, have also been implicated. These exposures may induce chronic inflammation or DNA damage, contributing to malignant transformation in pancreatic tissue [9].
Identifying individuals at elevated risk is crucial for implementing targeted screening and prevention strategies. High-risk populations include those with a strong family history of pancreatic cancer, defined as having two or more first-degree relatives affected. Familial pancreatic cancer (FPC) accounts for a significant subset of cases, even in the absence of known genetic syndromes (UpToDate, 2023). Several hereditary syndromes are associated with markedly increased risk. For example, individuals with hereditary breast and ovarian cancer syndrome (HBOC) due to BRCA mutations, Lynch syndrome (MLH1/MSH2), Peutz-Jeghers syndrome (STK11), and familial atypical multiple mole melanoma (CDKN2A) are all considered high-risk [8]. These syndromes often present with early-onset disease and may benefit from surveillance programs such as endoscopic ultrasound or MRI-based screening.
Clinical Presentation and Diagnosis of Pancreatic Cancer
Pancreatic cancer is among the deadliest malignancies, often diagnosed at an advanced stage due to its subtle and non-specific symptoms. Despite technological advances, the five-year survival rate remains dismally low, emphasizing the need for earlier detection and improved diagnostic strategies [10]. The pancreas, located deep in the abdomen behind the stomach, plays a crucial role in digestion and blood sugar regulation. Its hidden location contributes to the vague nature of early symptoms, which are often mistaken for benign gastrointestinal issues.
Figure 1:Anatomical location of the pancreas and its proximity to vital structures in the body. Source: Mayo Clinic [11].
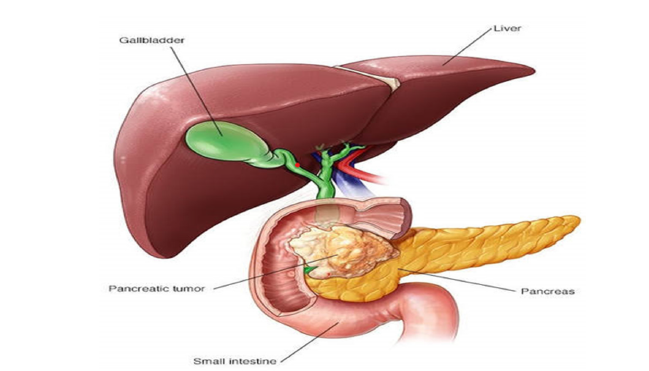
Patients frequently report abdominal pain or back discomfort, especially when tumours press against surrounding nerves. Figure 1 below illustrates the anatomical location of the pancreas and its proximity to vital structures such as the bile duct and duodenum.
This helps explain why tumours in the pancreatic head often cause jaundice, a yellowing of the skin and eyes due to bile duct obstruction [11]. Pancreatic ductal adenocarcinoma develops in the pancreas most commonly in the head where tumour growth and ductal obstruction cause early symptoms such as jaundice, dark urine, pale stools and pruritus due to impaired bile flow. As the tumour enlarges or occurs in the body/tail, it compresses nearby nerves and organs, producing deep epigastric or back pain. Tumour-induced pancreatic insufficiency leads to steatorrhea, malabsorption and weight loss, while systemic inflammatory and metabolic effects contribute to anorexia, fatigue and cachexia. Venous invasion or hypercoagulability from tumour-secreted procoagulants may cause migratory thrombophlebitis (Trousseau’s sign). Advanced disease often spreads to the liver, lungs, or peritoneum, worsening abdominal distension and causing ascites. These symptoms reflect both the pancreas’s central digestive role and the tumour’s aggressive, infiltrative nature (Eloubeidi et al., 2004).
Diagnosing pancreatic cancer is notoriously difficult. The disease often mimics benign conditions such as gallstones or gastritis, leading to misdiagnosis and delayed treatment. Figure 2 below highlights the diagnostic journey many patients face, often involving multiple consultations before reaching a definitive diagnosis [12].
Figure 2:Challenges encountered in diagnostic procedure of pancreatic cancer. Source: Primavesi [13].
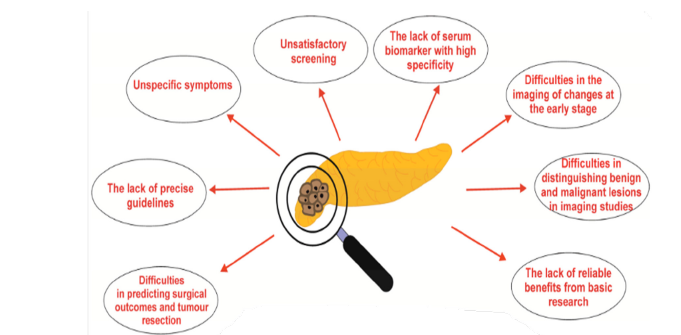
The absence of specific early symptoms and lack of effective screening tools further complicate diagnosis. Most cases are identified at stage III or IV, when curative surgery is no longer viable [13]. Even when symptoms are present, they are frequently dismissed or attributed to aging or lifestyle factors [14]. Imaging is central to the diagnosis, staging and management of pancreatic cancer. Each modality offers unique strengths depending on the clinical context.
Computed Tomography (CT)
Figure 3:A mass in the pancreatic head compressing nearby vessels revealed by a CT imaging. Source: Lee and Yoon [15].
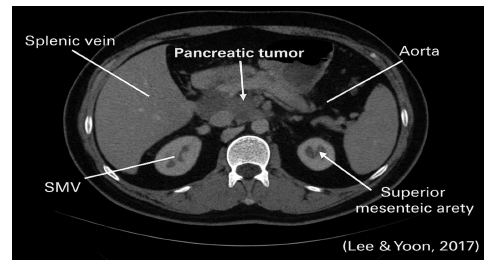
Computed Tomography (CT) is the first-line imaging technique. It provides high-resolution images of tumour size, vascular involvement and metastasis. Figure 3 shows a CT scan of a pancreatic tumour, revealing its location and relationship to surrounding vessels [15].
Magnetic Resonance Imaging (MRI)
Magnetic Resonance Imaging (MRI) offers superior soft tissue contrast and is especially useful for evaluating ductal anatomy. MRCP (Magnetic Resonance Cholangiopancreatography) can visualize bile and pancreatic ducts non-invasively. Figure 4 below, shows the Axial T1-weighted contrast-enhanced MRI demonstrating a hypointense mass in the pancreatic head region with ill-defined margins and delayed enhancement, consistent with pancreatic ductal adenocarcinoma. The lesion shows encasement of adjacent vascular structures, suggesting locally advanced disease. MRI provides superior soft tissue contrast and is valuable for assessing tumor extent, vascular involvement and resectability (Manfredi et al., 2005).
Figure 4:MRI Imaging of pancreatic adenocarcinoma. Source: Manfredi et al. (2005).
Positron Emission Tomography–CT (PET-CT)
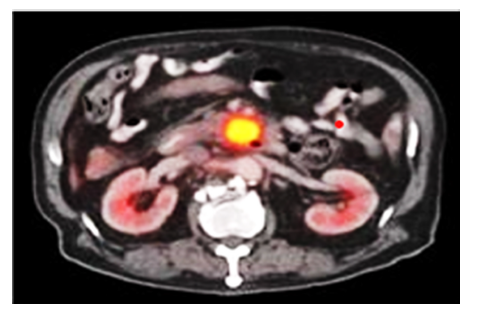
Positron Emission Tomography–CT (PET-CT) detects metabolic activity using 18F-FDG, identifying malignant lesions before structural changes are visible. It is particularly useful for staging and detecting distant metastases [4]. Axial PET/CT scan demonstrating a hypermetabolic lesion in the pancreatic region in Figure 5 below, consistent with suspected pancreatic adenocarcinoma. The lesion exhibits intense 18F-FDG uptake, indicative of elevated metabolic activity. Bilateral kidneys are visualized with physiologic tracer excretion. A smaller adjacent focus suggests possible regional lymphatic involvement. These findings support further diagnostic evaluation via endoscopic ultrasound and tissue biopsy.
Figure 5:MRI scan of pancreatic tumour. Source: Jha and Bijan [4].
Ultrasound
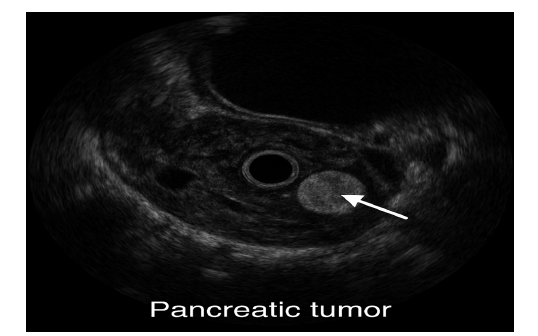
Ultrasound, especially Endoscopic Ultrasound (EUS) provides high-resolution images and allows fine-needle aspiration (FNA) to obtain tissue samples. It is highly sensitive for detecting small tumours and guiding biopsy procedures (Eloubeidi et al., 2004). Figure 6 revealed the Endoscopic ultrasound (EUS) image demonstrating a hypoechoic lesion within the pancreatic parenchyma, consistent with a suspected malignant neoplasm. The lesion exhibits irregular margins and heterogeneous echotexture, features commonly associated with pancreatic adenocarcinoma. EUS provides high-resolution imaging and facilitates fine-needle aspiration for histopathological confirmation.
Figure 6:Endoscopic ultrasound imaging of pancreatic cancer. Source: Eloubeidi et al. (2004).
Biomarkers and Liquid Biopsies
Due to the disease’s poor prognosis and well-known diagnostic difficulties, the search for trustworthy biomarkers for pancreatic cancer has accelerated recently. A promising approach to earlier identification, better monitoring, and individualized treatment plans is provided by biomarkers, which are biological substances that indicate disease processes [16]. To get around the drawbacks of the current diagnostic paradigms for pancreatic cancer, researchers are investigating both established blood indicators and new liquid biopsy technologies. Historically, carbohydrate antigen 19-9 (CA 19-9) has served as the cornerstone biomarker in pancreatic cancer management. It is a glycoprotein expressed by epithelial cells and released into the bloodstream by pancreatic tumours. Elevated CA 19-9 levels are often associated with advanced disease and can be used to monitor treatment response and disease progression [17]. However, its diagnostic utility is limited by low specificity and sensitivity, particularly in early-stage disease. CA 19-9 levels may also rise in benign conditions such as pancreatitis, cholestasis, and cirrhosis, and are undetectable in individuals lacking the Lewis antigen phenotype [18]. Carcinoembryonic antigen (CEA), another traditional marker, is less commonly used in pancreatic cancer but may complement CA 19-9 in assessing prognosis and recurrence risk [16].
The limitations of these conventional biomarkers have catalyzed interest in liquid biopsy technologies, which offer a noninvasive alternative for tumour profiling. Liquid biopsies analyze tumour-derived components in bodily fluids most commonly blood including circulating tumour DNA (ctDNA), circulating tumour cells (CTCs), and exosomes. Since these elements may be sampled often over time and represent the tumour’s genetic landscape, they allow for dynamic tracking of the course of the disease and the effectiveness of treatment [19].
Circulating tumour DNA, fragmented genetic material shed by cancer cells into the bloodstream, has emerged as a particularly promising biomarker. ctDNA can reveal tumour-specific mutations such as KRAS and TP53, which are prevalent in pancreatic ductal adenocarcinoma [20]. The detection of ctDNA allows for molecular characterization without the need for invasive tissue biopsies, and its levels correlate with tumour burden and therapeutic efficacy. Similarly, exosomes small extracellular vesicles containing proteins, lipids, and nucleic acids carry molecular signatures of the parent tumour and have demonstrated potential in early detection and prognosis [16].
The clinical applications of these emerging biomarkers extend beyond diagnosis. In high-risk individuals, such as those with familial pancreatic cancer or known genetic mutations, liquid biopsies may facilitate earlier detection before radiographic abnormalities appear. Moreover, in patients undergoing treatment, serial monitoring of ctDNA or exosomal content can provide realtime insights into therapeutic response and resistance mechanisms. This dynamic surveillance is particularly valuable in guiding adjustments to chemotherapy or targeted therapies, thereby enhancing personalized care [21].
Notwithstanding its potential, liquid biopsy, methods have issues with cost-effectiveness, sensitivity, and uniformity. Detection is made more difficult by the variety of pancreatic tumours and the scarcity of tumour-derived material in the bloodstream, particularly in the early stages of the disease. However, continuous developments in bioinformatics and sequencing technologies are progressively enhancing these instruments’ precision and therapeutic usefulness.
Pathological Features and Classification of Pancreatic Cancer
Pancreatic ductal adenocarcinoma accounts for approximately 90% of all pancreatic malignancies and arises from the epithelial lining of the pancreatic ducts. Histologically, PDAC is characterized by gland-forming malignant cells embedded in a dense desmoplastic stroma. These tumours exhibit marked cellular atypia, frequent mitoses and perineural invasion, contributing to their aggressive nature and poor prognosis [22]. In contrast, pancreatic neuroendocrine tumours represent a smaller subset, comprising 3–5% of cases. These neoplasms originate from neuroendocrine cells, are classified into well-differentiated PanNETs and poorly differentiated pancreatic neuroendocrine carcinomas (PanNECs). PanNETs typically display an organoid architecture with rosettes, trabeculae, and nests of uniform cells, while PanNECs resemble small or large cell carcinomas and are associated with high-grade histology and rapid progression [23] (Figure 7).
Figure 7:Histological large duct pattern classification of pancreatic ductal adenocarcinoma. Source: Lee et al. (2024).
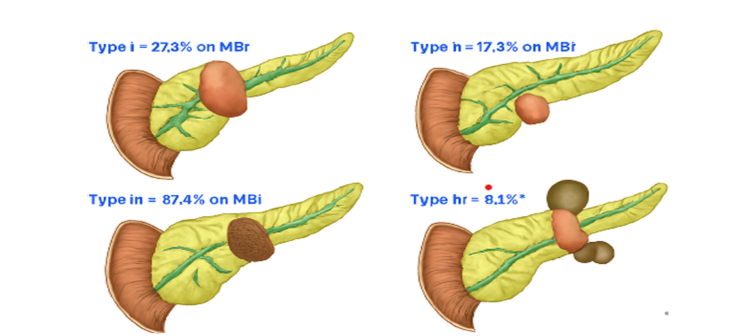
The imaging classification of pancreatic ductal adenocarcinoma (PDAC) with histological large duct pattern is divided into four types based on MRI features and ductal morphology. Type I (27.3%) shows a well-defined, round lesion near the pancreatic head, indicating localized ductal expansion with minimal invasion (Lee et al., 2024). Type II (17.3%) presents a smaller lesion in the body of the pancreas, reflecting early ductal changes with subtle imaging findings (Lee et al., 2024). Type III (87.4%) the most common displays a large, irregular mass in the pancreatic body, consistent with extensive ductal involvement and aggressive pathology (Lee et al., 2024). Type IV (8.1%) features multifocal lesions in the body and tail, suggesting diffuse ductal transformation and possible multicentric origin (Lee et al., 2024).
At the molecular level, pancreatic cancer is driven by a constellation of genetic alterations that influence tumour initiation, progression and therapeutic response. KRAS mutations are the most prevalent, occurring in approximately 88% of PDAC cases. These mutations lead to constitutive activation of the RAS/MAPK pathway, promoting uncontrolled cell proliferation and resistance to apoptosis [24]. TP53, a critical tumour suppressor gene, is mutated in nearly 77% of cases, resulting in impaired DNA damage response and genomic instability. SMAD4, involved in TGF-β signaling, is inactivated in about 29% of PDACs and is associated with poor prognosis and metastatic potential. The co-occurrence of these mutations defines the molecular landscape of PDAC and underscores its aggressive phenotype.
In PanNECs, molecular profiling reveals a striking overlap with PDAC, including frequent mutations in KRAS and TP53, suggesting a shared oncogenic pathway despite divergent histological features. Conversely, well-differentiated PanNETs exhibit distinct genetic alterations, such as mutations in MEN1, DAXX and ATRX, which are rarely seen in PDAC or PanNEC [22]. These molecular distinctions have profound implications for targeted therapy and personalized medicine, as emerging treatments increasingly rely on genomic signatures to guide intervention.
Management of Pancreatic Cancer
The management of pancreatic cancer remains one of the most formidable challenges in oncology due to its aggressive nature, late presentation, and limited therapeutic window. A multidisciplinary approach encompassing surgery, chemotherapy and radiation therapy is essential for optimizing outcomes [16]. Treatment strategies are tailored to disease stage, tumour location, patient performance status and molecular profile.
Surgical resection offers the only potential for cure in pancreatic cancer, yet only 15–20% of patients present with resectable disease at diagnosis. The most commonly performed procedure is the Whipple procedure (pancreaticoduodenectomy), indicated for tumours located in the head of the pancreas [25]. This complex operation involves the removal of the pancreatic head, duodenum, gallbladder, and part of the stomach, followed by reconstruction of the gastrointestinal tract to restore digestive continuity [2,26]. Despite its curative intent, the Whipple procedure carries significant morbidity, including delayed gastric emptying and pancreatic fistulas, and requires meticulous postoperative care. For tumours in the body or tail of the pancreas, a distal pancreatectomy is preferred. This procedure entails removal of the distal pancreas and often the spleen, given their anatomical proximity. Compared to the Whipple procedure, distal pancreatectomy is less invasive and associated with a shorter recovery period, although it may still result in endocrine and exocrine insufficiency [26]. In rare cases of diffuse pancreatic involvement, a total pancreatectomy may be performed, necessitating lifelong insulin and enzyme replacement therapy.
Chemotherapy plays a pivotal role across all stages of pancreatic cancer. In the adjuvant setting, chemotherapy is administered postoperatively to eradicate residual microscopic disease and reduce recurrence risk. Regimens such as modified FOLFIRINOX or gemcitabine-based combinations have demonstrated improved survival, with median overall survival extending up to 54 months in select cohorts [25]. Neoadjuvant chemotherapy, administered prior to surgery, is increasingly employed for borderline resectable or locally advanced tumours. This approach aims to downstage tumours, increase the likelihood of margin-negative resection, and identify patients with aggressive disease who may not benefit from surgery. Neoadjuvant therapy also allows for early systemic treatment of micrometastases, which are often present at diagnosis [27]. In advanced or metastatic disease, palliative chemotherapy remains the cornerstone of management. Agents such as gemcitabine, 5-fluorouracil, and nab-paclitaxel are used to alleviate symptoms, prolong survival, and maintain quality of life. While not curative, palliative chemotherapy can significantly improve functional status and reduce tumour-related complications [28].
Radiation therapy complements surgical and chemotherapeutic strategies, particularly in locally advanced or unresectable pancreatic cancer. External beam radiation therapy (EBRT) delivers high-energy x-rays to the tumour site, often in combination with chemotherapy (chemoradiation) to enhance cytotoxicity. EBRT is typically administered over several weeks and may be used in both neoadjuvant and adjuvant settings (American Cancer Society, 2024).
A more recent innovation is stereotactic body radiation therapy (SBRT), which delivers highly focused, high-dose radiation over a shorter duration typically five sessions within one to two weeks. SBRT offers superior precision, minimizing damage to surrounding tissues and reducing treatment-related morbidity [29]. It is particularly beneficial for patients with localized tumours who are not surgical candidates or those with borderline resectable disease requiring tumour shrinkage prior to surgery [30]. SBRT’s ability to track tumour movement during respiration and its use of fiducial markers for targeting have significantly improved treatment accuracy. Moreover, its shorter treatment course enhances patient convenience and may reduce the need for prolonged chemotherapy. However, careful patient selection is critical to avoid complications such as gastrointestinal ulceration, especially when tumours are adjacent to the bowel or stomach [1].
The integration of surgery, chemotherapy, and radiation therapy requires careful coordination among oncologists, surgeons, and radiologists. Treatment sequencing whether surgery precedes or follows systemic therapy depends on tumour resectability, patient fitness, and molecular markers. Increasingly, molecular profiling is guiding therapeutic decisions, with trials exploring targeted agents and immunotherapies in combination with conventional modalities [31,32].
Emerging Therapeutic Approaches
Targeted therapies have gained momentum as a precisionbased strategy to disrupt key oncogenic pathways in PDAC. Among these, KRAS has emerged as a central focus, given its mutation in over 90% of cases. Historically considered “undruggable,” KRAS has recently become targetable through small-molecule inhibitors such as sotorasib and adagrasib, which selectively bind to the KRASG12C mutant and inhibit downstream signaling (Long et al., 2024). Although KRASG12C is less common in PDAC than in lung or colorectal cancers, ongoing trials are exploring broader KRAS-targeting strategies, including siRNA-based silencing and combination regimens with autophagy modulators and CDK4/6 inhibitors (Hoang and Tsang, 2025). Beyond KRAS, other molecular targets are being investigated, including NRG1 fusions, NTRK and ROS1 rearrangements; REt alterations, and the PRMT5/CDKN2A/ MAT2A axis. These rare but actionable mutations offer therapeutic opportunities for molecularly defined subgroups. Additionally, inhibitors of EGFR and Claudin18.2 are being evaluated for their ability to disrupt epithelial signaling and enhance chemosensitivity (Li et al., 2024). The integration of next-generation sequencing into clinical workflows has facilitated the identification of these targets, enabling personalized treatment plans based on tumour genomics.
Immunotherapy, once considered ineffective in PDAC due to its immunologically “cold” tumour microenvironment, is undergoing a renaissance through combination strategies. Immune checkpoint inhibitors, such as pembrolizumab and nivolumab, have shown modest efficacy in tumours with high microsatellite instability (MSI-H) or mismatch repair deficiency (MMR-D), but their broader application remains limited (American Cancer Society, 2024). To enhance immunogenicity, researchers are combining checkpoint blockade with cancer vaccines, adoptive cell therapies and stromaldepleting agents. One promising approach involves the GVAX vaccine, which uses irradiated pancreatic cancer cells engineered to secrete GM-CSF, thereby stimulating dendritic cell activation. When combined with checkpoint inhibitors and CD137 agonists, GVAX has demonstrated increased infiltration of cytotoxic T cells and prolonged disease-free survival in early-phase trials (Zheng et al., 2024). These findings suggest that priming the immune system prior to surgery may improve long-term outcomes and reduce recurrence.
Nanotechnology offers a transformative approach to drug delivery in pancreatic cancer, addressing the limitations of poor vascularization, high interstitial pressure, and chemoresistance. Nanoparticle-based systems can be engineered to enhance drug solubility, prolong circulation time, and facilitate targeted delivery to tumour cells. Lipid-based, polymeric, and inorganic nanoparticles have all been explored for their ability to penetrate the dense extracellular matrix and release therapeutic agents in a controlled manner (Jia et al., 2021). Recent innovations include pH-sensitive and reactive oxygen species (ROS)-responsive nanoparticles that release drugs in response to the acidic and oxidative conditions of the tumour microenvironment. These smart carriers can bypass stromal barriers and deliver payloads directly to cancer cells, minimizing systemic toxicity. Additionally, multifunctional nanoparticles are being developed to co-deliver chemotherapy and immunomodulators, thereby enhancing both cytotoxic and immune-mediated effects (Viegas et al., 2023).
Anti-Aging Gene Sirtuin 1: Critical to Diagnosis, Treatment and Management of Pancreatic Cancer
Sirtuin 1 (SIRT1), a member of the sirtuin family of nicotinamide adenine dinucleotide (NAD⁺)-dependent deacetylases, has emerged as a pivotal regulator of cellular metabolism, longevity, and cancer biology. Originally identified as an anti-aging gene, SIRT1 influences a variety of biological processes including DNA repair, apoptosis, oxidative stress response and inflammation, all of which are central to cancer development and progression [33]. In the context of pancreatic cancer, one of the most lethal malignancies with a fiveyear survival rate below 12%, SIRT1 plays a multifaceted and paradoxical role that has significant implications for diagnosis, treatment and disease management.
At the molecular level, SIRT1 regulates the acetylation status of key transcription factors such as p53, NF-κB, FOXO, and HIF- 1α. This modulation allows SIRT1 to either suppress or promote tumorigenesis depending on the cellular context. In pancreatic ductal adenocarcinoma (PDAC), SIRT1 is often overexpressed, correlating with enhanced tumor growth, invasion, and chemoresistance. By deacetylating and inactivating p53, SIRT1 reduces apoptotic signaling, enabling tumor cells to survive under metabolic and oxidative stress conditions (Chen et al., 2021). Similarly, SIRT1- mediated activation of HIF-1α supports the adaptation of pancreatic cancer cells to hypoxic microenvironments, which is a hallmark of PDAC progression. Consequently, SIRT1 has been proposed as both a diagnostic biomarker and a potential therapeutic target in pancreatic cancer.
Recent studies indicate that elevated SIRT1 expression in pancreatic tumor tissues and serum may serve as a diagnostic and prognostic biomarker. Its overexpression is associated with poor clinical outcomes, high metastatic potential and reduced response to conventional chemotherapy. Techniques such as immunohistochemistry and quantitative PCR can be used to evaluate SIRT1 levels in tumor biopsies, potentially allowing for earlier diagnosis and patient stratification. Moreover, integrating SIRT1 expression profiling with other molecular markers such as KRAS and p16 mutations could improve diagnostic precision and guide personalized therapy [34].
Therapeutically, targeting SIRT1 offers promising avenues for treatment. Pharmacological inhibitors like sirtinol, cambinol and EX-527 have demonstrated efficacy in suppressing SIRT1 activity, leading to increased cancer cell apoptosis and reduced proliferation.
These inhibitors can be used in combination with standard chemotherapeutic agents such as gemcitabine to overcome drug resistance, a major challenge in pancreatic cancer management (Chen et al., 2021). Conversely, in some cellular contexts, activation of SIRT1 through compounds like resveratrol has shown anti-tumor effects by promoting metabolic reprogramming and reducing oxidative stress. Therefore, understanding the dual role of SIRT1 is essential for its safe and effective therapeutic targeting (Li et al., 2020).
In terms of management, modulation of SIRT1 activity could also enhance the efficacy of existing treatment modalities, including immunotherapy and radiation therapy. By regulating cellular stress responses, SIRT1 influences the tumor microenvironment and the immune landscape of pancreatic cancer. Hence, combining SIRT1 modulators with immunotherapeutic approaches could improve patient outcomes by reducing tumor immune evasion and enhancing T-cell activity [34] (Hori et al., 2019).
Clinical Trials and Future Directions
Despite decades of study and clinical advancement, pancreatic cancer still has a five-year survival rate of about 12%, making it one of the most deadly cancers. Global efforts to investigate new treatment modalities through clinical trials and precision medicine have been sparked by the ongoing problems of late-stage diagnosis, therapeutic resistance, and tumour heterogeneity. In addition to increasing survival, these initiatives seek to change the therapeutic paradigm from widespread cytotoxicity to tailored treatment [35]. A wide range of therapeutic approaches, including as targeted medicines, immunotherapies and combination regimens intended to get around the drawbacks of traditional chemotherapy, are being studied in current clinical studies. Among the most promising developments is the use of KRAS inhibitors, such as adagrasib and MRTX1133, which target the KRASG12D mutation found in over 90% of pancreatic ductal adenocarcinomas [36]. These agents are being tested both as monotherapies and in combination with drugs like venetoclax, a BCL2 inhibitor, to enhance apoptosis and overcome resistance mechanisms [37].
Inhibitor of Apoptosis Protein (IAP) antagonists like APG- 1387, mTOR inhibitors like sirolimus and PARP inhibitors like olaparib for patients with BRCA mutations are being investigated in other trials. To increase effectiveness, these drugs are frequently used in conjunction with conventional chemotherapy treatments [38]. Combination techniques are being used to reexamine immunotherapy, which has long been thought to be unsuccessful in pancreatic cancer because of the tumour’s immunosuppressive microenvironment. Early results indicate that immunological response can be improved by using immune checkpoint inhibitors (such as durvalumab and tremelimumab) in combination with chemotherapy or in situ vaccination methods [39]. A move toward multi-modal approaches that address tumour biology and the surrounding milieu is also seen in the investigation of stromaltargeting drugs like minnelide, metabolic inhibitors, and mRNA vaccines [31,32]. These trials represent a critical step toward expanding the therapeutic arsenal and improving outcomes for patients with advanced disease.
The future of pancreatic cancer treatment lies in precision medicine, a paradigm that tailors therapy based on individual genetic, molecular and clinical profiles. Genomic profiling has revealed key mutations KRAS, TP53, CDKN2A, SMAD4 that not only drive tumourigenesis but also serve as potential therapeutic targets [40]. High-throughput sequencing and liquid biopsy technologies now enable real-time monitoring of tumour evolution, allowing clinicians to adapt treatment strategies dynamically. Precision medicine also encompasses transcriptomic and proteomic analyses, which help stratify patients into molecular subtypes with distinct prognostic and therapeutic implications. For example, patients with homologous recombination deficiency (HRD) may benefit from DNA damage response-targeted therapies, while those with microsatellite instability may respond to immunotherapy [41]. The integration of artificial intelligence and machine learning into clinical decision-making further enhances the ability to predict treatment response and optimize care. Personalized therapy is not limited to drug selection; it also involves tailoring surgical and radiotherapeutic approaches. For instance, stereotactic body radiation therapy (SBRT) may be preferred in patients with localized disease and poor surgical candidacy, while neoadjuvant chemotherapy may be prioritized in borderline resectable tumours with aggressive molecular signatures [42].
Despite these advances, significant challenges persist. The tumour microenvironment in pancreatic cancer is notoriously desmoplastic and immunosuppressive, limiting drug penetration and immune activation. The lack of reliable early detection biomarkers remains a major barrier, as most patients are diagnosed at advanced stages when curative options are limited [43]. Moreover, therapeutic resistance both intrinsic and acquired continues to undermine long-term efficacy, necessitating the development of adaptive treatment strategies [44].
Opportunities lie in the integration of multi-omics data, which can unravel the complex interplay between genetic mutations, epigenetic modifications, and metabolic pathways. Collaborative initiatives such as the “Know Your Tumour” program have demonstrated the feasibility of large-scale molecular profiling and its impact on treatment selection [45]. Furthermore, expanding access to clinical trials and fostering global research networks can accelerate the translation of laboratory discoveries into clinical practice [46-57].
Conclusion
Pancreatic cancer remains one of the most formidable challenges in oncology, marked by late-stage diagnosis, rapid progression, and limited therapeutic options. This manuscript has explored the multifaceted landscape of pancreatic cancer, from its pathological features and molecular drivers such as KRAS, TP53, and SMAD4 mutations to evolving diagnostic tools like liquid biopsies and circulating tumour DNA. Surgical resection, particularly the Whipple procedure and distal pancreatectomy, continues to offer the best chance for cure in resectable cases, while chemotherapy and radiation therapy serve as critical adjuncts across disease stages. Recent advances in clinical trials, including targeted therapies and immunomodulatory agents, reflect a growing shift toward biologically driven treatment paradigms. Precision medicine, informed by genomic and proteomic profiling, is redefining therapeutic strategies and enabling more personalized care.
The implications for clinical practice are profound. Integrating molecular diagnostics into routine care can enhance early detection, guide treatment selection, and improve prognostic accuracy. The emergence of neoadjuvant approaches and stereotactic body radiation therapy has expanded surgical candidacy and improved local control. Yet, challenges persist particularly in overcoming therapeutic resistance and addressing the immunosuppressive tumour microenvironment. Future research must prioritize the development of robust biomarkers, innovative trial designs, and equitable access to cutting-edge therapies. By embracing a multidisciplinary and data-driven approach, the oncology community can move closer to transforming pancreatic cancer from a terminal diagnosis into a manageable disease, ultimately improving survival and quality of life for patients worldwide.
Acknowledgments
We want to thank all the researchers who contributed to the success of this research work.
Conflict of Interest
The authors declared that there are no conflicts of interest.
Funding
No funding was received for this research work.
References
- Cancer Research UK (2024) Radiotherapy for pancreatic cancer.
- Pancreatic Cancer Action Network (2024) Whipple procedure (pancreaticoduodenectomy).
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, et al. (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 71(3): 209-249.
- Jha P, Bijan B (2015) PET/CT for pancreatic malignancy: Potential and pitfalls. Journal of Nuclear Medicine Technology 43(2): 92-97.
- World Cancer Research Fund (2022) Pancreatic cancer statistics.
- Zeng L, Zho W, Yang J, Yu Z, Chen R (2023) Genetic risk and lifestyle in pancreatic cancer. BMC Medicine 21: 489.
- American Cancer Society (2023) Pancreatic cancer risk factors.
- Pan CAN (2023) Genetics and hereditary factors of pancreatic cancer.
- Tsai HJ, Chang JS (2019) Environmental risk factors of pancreatic cancer. Journal of Clinical Medicine 8(9): 1427.
- American Cancer Society (2025) Signs and symptoms of pancreatic cancer.
- Mayo Clinic (2025) Pancreatic Cancer-Symptoms and causes.
- Singh P (2025) Understanding pancreatic cancer detection challenges. PhysicsCore.
- Primavesi F (2021) Clinical presentation and symptoms in pancreatic cancer. In Textbook of Pancreatic Cancer, pp. 357-368.
- Parham R (2024) Understanding pancreatic cancer diagnosis: Challenges, advances and hope. Journal of Cancer Diagnosis 8(2).
- Lee JM, Yoon JH (2017) Imaging diagnosis of pancreatic cancer: CT and MRI. In Pancreatic Cancer, pp. 95-114.
- Hussein AD, Al-Shammari MJI, Karim Alqaisy MR, Mohsein OA (2025) Emerging biomarkers in cancer detection and prognosis: A comprehensive review. International Journal of Immunology Sciences 7(1): 1-12.
- Demarzo MG, Facchini C, Bisso GR, Marrone C, Parodi MC (2024) Traditional biomarkers in patients with pancreatic cancer staged by CT and EUS: Is there still a role in the molecular era? Gastrointestinal Disorders 6(3): 733-741.
- Medical News Today (2024) Tumour markers for pancreatic cancer: CA 19-9 and CEA.
- Yin H, Zhang M, Zhang Y, Zhang X, Zhang X, et al. (2025) Liquid biopsies in cancer. Molecular Biomedicine 6(1): 18.
- Jamal MH, Porel P, Aran KR (2025) Emerging biomarkers for pancreatic cancer: From early detection to personalized therapy. Clinical and Translational Oncology 27(11) 4071-4090.
- Vitale F, Zileri DL, Paratore M, Negri M, Nista EC, et al. (2024) The past, present and future of biomarkers for the early diagnosis of pancreatic cancer. Biomedicines 12(12): 2840.
- Gao HL, Wang WQ, Yu XJ, Liu L (2020) Molecular drivers and cells of origin in pancreatic ductal adenocarcinoma and pancreatic neuroendocrine carcinoma. Exp Hematol Oncol 9: 28.
- El-Sharkawy SL, Badawi MA, Abdelaal WE, Abbas NF (2025) Overview of the histopathological and immunohistochemical characteristics of neuroendocrine neoplasms. Bulletin of the National Research Centre.
- Stefanoudakis D, Frountzas M, Schizas D, Michalopoulos NV, Drakaki A, et al. (2024) Significance of TP53, CDKN2A, SMAD4 and KRAS in pancreatic cancer. Curr Issues Mol Biol 46(4): 2827-2844.
- Maeda S, Unno M, Yu J (2019) Adjuvant and neoadjuvant therapy for pancreatic cancer. Journal of Pancreatology 2(3): 100-106.
- Apollo Hospitals (2025) Understanding surgical options in pancreatic cancer.
- Hammad AY, Hodges JC, AlMasri S, Paniccia A, Lee KK, et al. (2023) Evaluation of adjuvant chemotherapy survival outcomes among patients with node-negative disease after neoadjuvant therapy. JAMA Surgery 158(1): 55-62.
- Birk D, Beger HG (2001) Neoadjuvant, adjuvant, and palliative treatment of pancreatic cancer. Current Gastroenterology Reports 3(2): 129-135.
- Mfon NP, Obi BE, Chuwang MC, Nkemehule F, Oladosu MA, et al. (2025) Evaluation of the effects of cannabis sativa on the body weight and uterus of female albino rats. Trends in Medical Research 20(1): 44-49.
- MD Anderson Cancer Center (2018) Stereotactic body radiation therapy: A new option for pancreatic cancer patients Cancer wise.
- Anih DC, Arowora AK, Abah MA, Ugwuoke KC (2025) Biochemical effects of microplastics on human health: A comprehensive review. Science International 13(1): 27-34.
- Anih DC, Arowora KA, Ugwuoke KC, Abah MA, Habibu B (2025) Nutritional modulation of epigenetic changes induced by mycotoxins: A biochemical perspective for at risk populations in Africa. Science International 13(1): 90-109.
- Li L (2021) SIRT1 and pancreatic cancer: Current insights into its role and therapeutic potential. Frontiers in Oncology 11: 672390.
- Hori Y, et al. (2019) SIRT1 expression predicts poor prognosis in pancreatic ductal adenocarcinoma and correlates with epithelial–mesenchymal transition. Cancer Science 110(6): 2080-2092.
- David Chinonso Anih, Kayode Adebisi Arowora, Moses Adondua Abah, Kenneth Chinekwu Ugwuoke, Bilyaminu Habibu (2025) Redefining biomolecular frontiers: The impact of artificial intelligence in biochemistry and medicine. Journal of Medical Sciences 25: 1-10.
- Shenoy A, Yousif A, Hussain MD (2025) Recent advances and challenges in the treatment of advanced pancreatic cancer.
- Munshi HG (2024) Combination therapy may improve treatment response in pancreatic cancer.
- Saad A, Ameen OA, Ahmed FA (2025) Antidiabetic effect of chrysophyllum albidum pulp extract on streptozotocin-induced diabetic rats. Curre Res Diabetes & Obes J 18(1): 555976.
- Mohammad H (2023) Beyond chemotherapy: Exploring synergistic combinations in pancreatic cancer treatment. Journal of Cancer Clinical Trials 8: 6.
- Moussa R (2024) Precision medicine in pancreatic cancer: Current challenges and future directions. Clinical Gastroenterology Journal 9(1).
- Besong E Obi, Akpotuzor D Uchendu, Shella O Besong, Anthony N Kokelu, Moses A Abah, et al. (2025) Carcinoembryonic antigen levels and some coagulation parameters of breast cancer patients attending university of Calabar teaching hospital. Journal of Cancer Management and Research, BioRes Scientia Publishers 3(1): 1-15.
- Moses Adondua Abah (2025) Effect of green synthesized silver nanoparticles from orange peels on growth performance and liver function parameters of male albino rats. Pri Mera Scientific Surgical Research and Practice 5(5): 3-14.
- Batey M (2019) Challenges and opportunities in pancreatic cancer research.
- Victoria IA, Moses AA, Ale EM, Lyambee KQ, Victor O, et al. (2025) Effect of cucumis callosus fruit extract on the kidney function of DMBA-induced mammary cancer in female albino wistar rats. Canc Therapy & Oncol Int J 28(4): 556245.
- Keane F, Park W, O’Reilly EM (2022) Precision approaches to pancreatic cancer therapy: What now and what next? Current Treatment Options in Gastroenterology 20: 406-428.
- Ale EM, Kade IJ, Timothy MJ, Boyi RHN, Asuelimen SO, et al. (2025) Assessment of the participation of sulfhydryl proteins in the glutathione peroxidase mimicry of diphenyl diselenide in the presence of thiol alkylating agent. Sci Rep 15(1): 17482.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, et al. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6): 394-424.
- Chen J, et al. (2020) The dual role of SIRT1 in cancer: Tumor suppressor and oncogene. Frontiers in Bioscience 25(2): 437-452.
- Hirshberg Foundation (2023) Family genetics in pancreatic cancer and high-risk individuals.
- Hruban RH, Adsay NV, Albores-Saavedra J, Compton C, Garrett ES, et al. (2000) Pancreatic intraepithelial neoplasia: A new nomenclature and classification system for pancreatic duct lesions. American Journal of Surgical Pathology 25(5): 579-586.
- Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, et al. (2016) Pancreatic cancer. Nature Reviews Disease Primers 2: 16022.
- Neuzillet C, Tijeras Raballand A, Ragulan C, Cros J, Patil Y, et al. (2015) Inter- and intra-tumoural heterogeneity in pancreatic ductal adenocarcinoma: Neoplastic cell and stroma profiling. Journal of Pathology 236(2): 211–224.
- Radiopaedia (2024) Pancreatic cancer staging.
- Rawla P, Sunkara T, Gaduputi V (2019) Epidemiology of pancreatic cancer: Global trends, etiology and risk factors. World Journal of Oncology 10(1): 10-27.
- Siegel RL, Miller KD, Hannah EF, Jemal A (2021) Cancer statistics, 2021. CA Cancer J Clin 71(1): 7-33.
- Vincent A, Herman J, Schulick R, Hruban RH, Goggins M (2011) Pancreatic cancer. The Lancet 378(9791): 607-620.
- Wolfgang CL, Herman JM, Laheru DA, Klein AP, Erdek MA, et al. (2013) Recent progress in pancreatic cancer. CA: A Cancer Journal for Clinicians 63(5): 318-348.
© 2025. Moses Adondua Abah. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






