- Submissions

Full Text
Modern Research in Dentistry
Managements of Immature Apex: a Review
Kareem A Mohamed Kamel1* and Rasha M Abuzied2
1Former Dental Regional Adviser, Head of Royal Dentists Institute, Egypt
2Restorative Dentistry Department, Royal Dentists Institute, Egypt
*Corresponding author: Kareem A Mohamed Kamel, Dental Regional Adviser, Royal Dentists Institute, 6th of October City, Cairo, Egypt
Submission: July 08, 2017; Published: October 20, 2017

ISSN:2637-7764Volume1 Issue1
Abstract
This paper reviews the rationale and techniques for treatment of the non-vital immature tooth. The importance of careful case assessment and accurate pulpal diagnosis in the treatment of immature teeth with pulpal injury cannot be overemphasized. The treatment options starts from indirect pulp capping, direct pulp capping, partial pulpotomy, full pulpotomy, apex genesis, apexification and ending up with revitalization. Describing the techniques, indications and the drawbacks of each treatment option.
Root Development
Root development commences after the completion of enamel formation. The cells of inner and outer enamel epithelia unite at point forming cervical loop, begin to proliferate and form a structure known as the Hertwig epithelial sheath. This sheath determines the size and shape of root/s of the tooth [1]. Apical root closure is completed approximately 2-3 years after tooth eruption [2].
Causes of Pulpal Disease
The major irritants of pulp tissue include various bacteria, trauma, dental procedures generating thermal stimulation and chemical agent. Irritation of pulp tissue results in major changes in pulp microcirculation that can lead to pulp necrosis and arrest root formation [3].
Dental caries is the most prevalent microbial infectious disease. As the caries lesion approximates the pulp, there is acute exacerbation of the precedent chronic inflammation characterized by an influx of neutrophils [4]. In the presence of severe inflammation, focal microabscesses form and eventually coalesce leading to progressive pulpal necrosis.
Restorative procedures can also affect pulpal tissue. Thermal injury may occur as result of tooth preparation or finishing procedures using dry cutting, dull burs, deep periodontal curettage, orthodontic movement, or lasers and air abrasion devices [5].
Chemical irritants of pulp include cavity cleansers such as alcohol, chloroform and hydrogen peroxide as well as some substance in restorative materials and cavity liner [6]. One key requirement of a successful restorative procedure is to cause minimal additional irritation of the pulp so as not to interfere with normal pulpal healing. Crown-fracture teeth with or without pulp exposure and associated luxation injury experience a greater frequency of pulp necrosis [7]. However immature permanent tooth has considerable capacity to heal after traumatic pulp exposure, so conservative pulp therapies are important to preserve pulp vitality and allow for continued root formation.
Clinical Tests to Determine Pulp Condition
Pulpal conditions and stage of root development are the major factors in the selection of treatment plan [8]. However with early diagnosis and intervention, pulp preservation strategies promote an environment for continued dentine apposition and root formation.
Diagnosis begins with a careful medical examination and any implications related to the anticipated treatment. A Thorough dental history including all symptoms and characteristics of associated pain is essential. Visual examination of both soft and hard tissue for the presence or absence of swelling, crown discoloration and caries.
Mobility and periodontal probing can provide information regarding the health of status pulp. Electric and thermal tests are of limited value due to their varied responses in permanent teeth with immature apex [9]. After traumatic injuries electric and thermal pulp tests may be unreliable, only generalized impressions may be gained from these tests [10].
Attempts have been made to use laser Doppler flowmetry (LDF) for measurement of blood flow in traumatized teeth as this would provide more accurate readings [11]. The pulse oximeter also offers accurate means of monitoring pulp vitality by recording the oxygenation of pulpal flow [12].
Radiographic examination of teeth requires good quality periapical and bitewing radiographs. These radiographs reveal the status of periapical tissues, presence and proximity of pulpal caries and stage of root development. The use of cone beam computed tomography should provide more accurate information regarding the condition of periapical tissues and root formation compared to 2- dimension conventional radiographs [13].
However it is still difficult to determine if the pulp is reversibly or irreversibly affected [14]. Moreover children may have unreliable clinical symptoms and exaggerated responses to percussion, palpation and pulp tests that do not correlate well with histopathological condition of pulp in immature teeth [15].
A recent systematic review [16] aimed to appraise the diagnostic accuracy of sign/symptoms, pulp tests to determine the condition of pulp in teeth affected by deep caries, trauma and other types of injuries showed that there is insufficient evidence to assess the value of toothache or abnormal reaction to hot/cold stimulation for determining pulp condition. However it is hoped that by combining the result of history, examination and diagnostic tests, an accurate clinical diagnosis of pulp vitality can be made in most cases.
Treatment of Teeth with Vital Pulp and Open Apices
When pulp exposure occurs in immature tooth due to caries or trauma, the exposed pulp can heal if protected from further injury Treatment of immature teeth has changed dramatically in recent years as new concepts and materials have developed. Vital pulp therapies are the treatment of choice for traumatized and carious teeth with vital pulps and open apices [17]. The approaches include indirect pulp capping in deep caries cavities and direct pulp capping or pulpotomy in cases of pulp exposure.
The key to the success of vital pulp therapy might partly be strict case selection and proper treatment protocol. Vital pulp therapy should not only be performed in teeth with signs and symptoms of reversible pulpitis [18]. This may be against the traditional school of thought that vital pulp therapy should only be performed in teeth reversible pulpitis [19]. Several studies included teeth with signs of irreversible pulpitis such as teeth with spontaneous pain [20-22], and teeth that were positive to percussion [20,21] were treated successfully. In full pulpotomy studies [22-24], teeth with periodical radiolucency lesions were treated successfully with weighted pooled success rate of 92% [25].
Moreover, histological studies demonstrate that curiously exposed vital pulp was not always completely infected. Occasionally the inflammation was localized adjacent to carious lesion, not spreading to the whole coronal or reticular pulp [25,26].
Apexogenesis
Apex=root end, genesis=formation. Apex genesis is defined as "a vital pulp therapy procedure to encourage continued physiological development and formation of the root end" [18]. Only inflamed pulp tissue should be removed and bioactive material is placed over remaining healthy pulp tissue.
The goals of apex genesis are [19]:
a) Allow continued development of root length.
b) Maintain pulp vitality, thus allow continued deposition of dentin.
c) Promoting root end closure, thus creating natural apical constriction.
d) Generating Dentine Bridge at the site of pulpotomy.
Treatment of Teeth with Necrotic Pulp and Open Apices
Incomplete root development can provide a challenging clinical situation in treatments [20].
A. Cleaning and shaping of blunderbuss canal is difficult.
B. Necrotic debris in wide root canal is difficult to completely disinfect.
C. Thin, fragile dentine walls are liable to fracture.
D. Risk of extending materials beyond apex.
Three techniques were reported to obdurate an immature tooth which involved the use of a root filling material without the induction of the apical closure [21].
a. Placement of large gutta-percha or customized guttapercha cone with sealer at the apex.
b. Placement of gutta-percha with sealer short of apex.
c. Periodical surgery.
However these techniques will not provide apical barrier. Another two techniques were reported aimed to provide apical barrier [21].
i. Placement of calcium hydroxide to induce mineralized apical barrier.
ii. Placement of biocompatible material such as dentine chips against which a root filling is placed.
iii. Until recently, the traditional approach to treat immature apex is apexification [22].
Apexification
Apexification is histological term defined as "a method to induce a calcified barrier in a root with open apex" [18]. The infected necrotic pulp is removed up to the apex by means of mechanical debridement and anti septic chemical irrigation [22].
Apical hard tissue barrier formation following apexification is reparative process of the dentine-pulp complex [23]. However, continued physiologic root development of immature permanent tooth with infected necrotic pulps and apical periodontitis after apexification procedure with calcium hydroxide was reported [24].
Contemporary Materials
Calcium hydroxide
Calcium hydroxide is the most popular material to induce normal root development [25]. Calcium hydroxide is white odorless powder and is chemically classified as strong base with PH 12.5; in contact with aqueous fluids it dissociate into calcium and hydroxyl ions [25].
The antimicrobial activity of calcium hydroxide is related to the release of highly reactive hydroxyl ions in aqueous environment which mainly affect cytoplasm membranes, proteins and DNA of bacterial cells [26].
The mineralizing action of calcium hydroxide is directly influenced by its high PH. The alkaline PH not only neutralizes acids from osteoclasts but also activate alkaline phosphates enzyme which plays a role in hard tissue formation [27]. However, calcium ions present in Dentine Bridge originated from systemic circulation [28].
Calcium hydroxide is biocompatible, but unfortunately has low compressive stress that is not compatible with condensation forces of amalgam, also solubility of calcium hydroxide in fluids is a problem as a reliable seal cannot be achieved so a good coronal seal is required in case of vital pulp therapy [29].
Mineral trioxide aggregate
Mineral trioxide aggregate is bioactive material that influences its surrounding environment. MTA is currently marked in two forms: white and gray. MTA was introduced in gray but because of its discoloration potential, White MTA was developed [30]. Gray MTA basically consists of declaim, tricalcium silicate and bismuth oxide, whereas white MTA is composed of tricalcium silicate and bismuth oxide [31].
MTA is prepare by mixing its powder with sterile water in a 3:1 powder to liquid ratio that result in a colloid gel that solidifies into hard structure [32]. The PH value of MTA is 10.2 after mixing. This value rises to 12.5 at three hour [33].
The mean setting time of MTA is two hours and 45 minutes. At 24 hours MTA has the lowest compressive strength among materials (40MPa), but increased after 21 days (67MPa) [33]. A recent study reported that compressive strength of MTA is higher when mixed mechanically and placed by ultrasonic agitation than when mixed manually and placed without ultrasonic agitation [34].
The solubility of MTA is influenced by powder to water ratio. In fact, higher water to powder ratios increase MTA porosity and solubility [34]. MTA has an antibacterial and antifungal effect. Lowering the powder to liquid ratio might adversely affect the antibacterial and antifungal properties of MTA [35]. MTA has Cementoconductivity and cementoinductivity properties that have been confirmed when reasonable concentration of material (less than 20mg/ml) has been used. MTA offers a biological substrate for cement oblasts attachment and growth as well as production of mineralized matrix gene (osteocalcin) and protein expression over MTA [36].
Indirect Pulp Capping
Indirect pulp capping is "procedure in which a material is placed on thin partition of remaining carious dentine that if removed might expose the pulp in immature permanent teeth" [18]. Also called "stepwise technique".
Indications [37]
a. Permanent teeth.
b. Normal response to pulp tests.
c. No symptoms of pulpitis or with diagnosis of reversible pulpitis.
d. Deep caries lesion radio graphically with no apical pathosis.
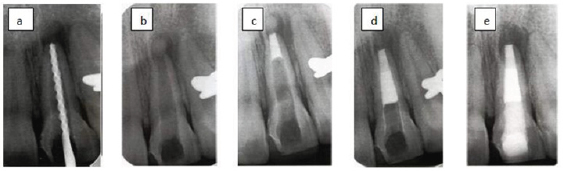
Technique (Figure 1)
This approach involves 2 steps process. The first step, the tooth is anesthetized and isolated with rubber dam, the carious dentine is removed completely from the dentin-enamel junction and only the softest infected dentine is excavated, leaving a carious layer over the pulp. Calcium hydroxide is then applied to the carious dentine. The objective is to change the cryogenic environment in order to decrease number of bacteria and close remaining caries from biofilm of the oral cavity. Subsequent radiographic reevaluation to ensure formation of Dentine Bridge and normal periodical status.
Figure 1: Cesare cesariano.
a) Preoperative radiograph of maxillary first molar showing deep occlusal caries.
b) Caries are removes except a layer immediately over the pulp.
c) CH is applies to caries dentine.
d) Postoperative radiograph after 1 year, the remaining caries are removes and permanent restoration is placed.
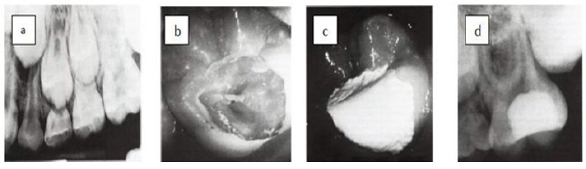
The second step, after 6 to 12 months allowing sufficient time for tertiary dentine formation, the carious lesion is reentered and the caries is removed to the formed dentine bridge and permanent restoration is placed [38]. However, what is critical to both steps of excavation is the placement of well sealed restoration [39].
One step indirect pulp capping was also reported [40], which involves minimal removal of caries and sealed with permanent filling restoration without a return for additional excavation. Two steps indirect pulp capping is also questioned of whether or not to have any advantage over one step treatment regimen. In fact, there may be two important disadvantages for the two steps technique. The first is the requirement of patient compliance and the assurance of return visit after 6-12 months. Secondly, a bacterial-tight seal is essential between visits. Thirdly, the two steps treatment is more expensive, requiring twice as much as operator and office time [41].
Mode of action
When thickness of remaining dentine including reactionary dentine between the front of bacterial penetration and the pulp is 1.1mm or more, the pulpal inflammatory response to bacterial infection of the dentinal tubules is negligible. However, when the bacterial penetration in the dentinal tubules reaches to within 0.5mm from the pulp, there is significant increase in the pulpal inflammation [42].
Indirect pulp capping intended to protect the primary odontoblast and the pulp from further injury. When the infected dentine is removed, the affected dentine can rematerialize and promote odontoblasts to form reactionary dentine formation at the pulp-dentine junction [43]. Reactionary dentin genesis is usually caused by milder injury of the dentin-pulp complex as slowly. The second step, after 6 to 12 months allowing sufficient time for tertiary dentine formation, the carious lesion is reentered and the caries is removed to the formed dentine bridge and permanent restoration is placed [38]. However, what is critical to both steps of excavation is the placement of well sealed restoration [39]. One step indirect pulp capping was also reported [40], which involves minimal removal of caries and sealed with permanent filling restoration without a return for additional excavation. Two steps indirect pulp capping is also questioned of whether or not to have any advantage over one step treatment regimen. In fact, there may be two important disadvantages for the two steps technique. The first is the requirement of patient compliance and the assurance of return visit after 6-12 months. Secondly, a bacterial-tight seal is essential between visits. Thirdly, the two steps treatment is more expensive, requiring twice as much as operator and office time [41]. Reactionary dentin genesis is usually caused by milder injury of the dentin-pulp complex as slowly progressive caries, cavity preparation and biomaterial used as indirect pulp capping agents [44]. Reactionary dentin genesis is secreted by functional up regulation of surviving primary odontoblasts, stimulated by signaling molecules such as growth factor proteins and bioactive molecules released from dentine matrix during injury to the dentin- pulp complex [44].
Treatment outcome
In a study [45] for long term the survival of indirect pulp treatment performed in primary and permanent teeth with clinically diagnosed deep caries, showed survival rate of 96% for 86 primary molars and 93% for 34 permanent molars, it was also found that glass monomer cement may be preferred to calcium hydroxide (CH) as it prevents micro leakage when compared with CH. Many studies [46] was reported to determine whether complete excavation of caries has any advantage over stepwise treatment in an attempt to avoid pulp exposure if possible. A critical review [47] of treatment of deep caries by complete excavation or partial removal indicate that complete removal of infected dentine approaching the pulp is not required for successful caries removal. However, in systematic review [48] of caries and pulp treatment from Cochrane Database indicate lack of randomized clinical trials to support incomplete removal of caries.
In another systematic review [49] from Cochrane Database indicate that partial removal of caries is preferable to complete caries removal in deep caries. The review also indicated that studies used one step indirect pulp therapy did not reported adverse consequences over the two steps indirect pulp therapy.
In a recent randomized clinical trial [50] comparing complete excavation of deep caries with stepwise excavation gave less success rate (62.4%) for complete excavation than stepwise excavation (74.1%).
Direct Pulp Capping
Direct pulp capping is defined as "treatment of exposed vital pulp by sealing the pulpal wound with material such as mineral trioxide aggregate or calcium hydroxide to facilitate the formation of reparative dentine and maintain pulp vitality" [18].
Indications [37]
a. Restorable permanent teeth.
b. Carious or traumatic exposure with vital pulp.
c. No symptoms of pulpitis or with diagnosis of reversible pulpits.
Technique (Figure 2 & 3)
The tooth is anaesthetized and isolated with rubber dam. All caries and undermined enamel are removed with low speed round carbide bur and excavator. Preferably, caries detector dye is applied for 10 seconds on dried caries dentine, and then all stained dentine is removed [51]. Bleeding will often occur with small exposures and can be controlled by placement of cotton pellet moisture with 3- 6% sodium hypochlorite for 10-20 seconds. 2% chlorhexidine or 0.9% saline can be also used to stop bleeding. None of these agents negatively affected pulp healing at 90 days [52]. This step can be repeated for 10 minutes. If homeostasis is achieved within 10 minutes hard setting calcium hydroxide is placed over pulpal tissue and sealed with permanent restoration [53]. If MTA will be as direct pulp capping agent, two steps approach will be taken. The first step, MTA should be mixed according to the manufacturer's instruction. MTA is placed directly over exposed pulp tissue and surrounding dentine. MTA should have at least 1.5mm thickness.
Figure 2: Direct pulp capping in lower premolar.
a) A premolar tooth capped directly with MTA. B) Histologic section after six months shows formation of dentine bridge.
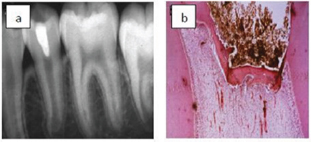
Figure 3: Direct pulp capping in lower molar. a) Preoperative radiograph shows extensive caries.
b) The decay was removed and pulp exposure was capped by MTA.

A circumferential region of dentine and enamel approximately 1.5mm should be cleared of MTA; this will provide adequate area for efficient seal of the restoration. Flat moist cotton is placed over entire MTA and strong interim restoration is placed. The second step will be after 1 to 10 days. The tooth is examined for any signs or symptoms and then interim restoration and cotton are removed. A definite restoration is placed. The patient is then recalled after 6 weeks when cold testing and symptoms are evaluated [54].
If bonded composite resin will be used immediately after placement of MTA, The unset MTA should be overlaid with a thin protective layer of resin modified glass monomer or flow able resin before bonding procedure [55].
Pulp capping with adhesive resin was reported in many studies [56-58]. However these studies revealed significant better result, less pulp necrosis and thicker dentine bridge when calcium hydroxide was used as direct capping agent.
Mode of action
At the site of exposure, primary odontoblasts together with other pulpal cells are destroyed and inflammation is initiated. If infection/inflammation in coronal pulp is under control, odontoblast-like cells will regenerate. Pulp capping materials such as MTA and calcium hydroxide induce the release of growth factor proteins and bio active molecules from dentin matrix. Postnatal stem cells in human dental pulp are capable of differentiating into odontoblast-like cells upon receiving inductive signals from bioactive molecules and growth factor proteins. Reparative dentin is formed from odontoblast-like cells and not from primary odontoblasts as in case of indirect pulp capping [59].
Treatment outcome
For decade, calcium hydroxide-base materials have been the gold standard for direct pulp capping [60,61]. A retrospective study [62] reported success rate of direct pulp capping with calcium hydroxide after 1 year (80.1%), 5 years (68%) and 9 years (58.7%). In another the long term study [63], the success rate of pulp capping with calcium hydroxide is 76.3% after 13.3 years. These results confirm that success rates using CH diminish over time, primarily due to the dissolution of material under restorations and over dentinal bridges with tunnel defects [64]. An alternative to calcium hydroxide-based capping materials is MTA, which was developed by Torabinejad and co-workers at Loma Linda University in the 1990s [65]. High success rate (97.9%) after capping with MTA in curiously exposed permanent teeth and all teeth showed continued root formation [66].
In clinical assessment of carious permanent teeth capped with MTA and followed for 6, 12, 18, 24 months, showed success rate (93%) with evidence of continued root growth [67]. Many case reports and clinical studies compared treatment outcome of MTA and CH when used as direct pulp capping agents. In preliminary study [68], 14 intact third maxillary molars teeth were capped with either CH or MTA. Histological examination of teeth showed dentinal bridge formation and mild chronic inflammation 2 months after pulp capping with MTA. While with CH, irregular and thin dentinal bridge was formed after 3 months with associated pulpal necrosis, hyperemia and inflammation.
Another histological study [69] revealed that all teeth capped with MTA showed dentinal bridge formation. In contrast, only 60% of CH-capped pulps showed hard tissue formation. Also MTA-capped pulps showed significantly thicker dentine bridge than those capped with CH. These results are similar to findings in another histological [70] that MTA-capped teeth were free from inflammation and showed dentine bridge formation within 3 months, while CH-capped teeth showed less consistent formation of Dentine Bridge that had numerous tunnel defects and persistence inflammation up to 3 months after capping.
A recent study [71] showed MTA is more effective than CH for maintaining long term pulp vitality after direct pulp capping. A recent systematic review [72] for vital pulp therapy in vital permanent teeth with cariously exposed pulp revealed success rate of direct pulp capping was different depending on follow up time (>6 months -1 year, 87.5%), (>1-2years, 95.4%), (>2-3 years, 87.7%) and (>3 years, 72.9%). Also showed that direct comparisons of weighed pooled success rate when using either CH or MTA as pulp capping medication revealed no statistically significant difference, while in indirect comparisons of weighted pooled success rate showed that MTA was superior to calcium hydroxide in direct pulp capping.
A recent retrospective study [55] aimed to evaluate success rate and treatment outcome of direct pulp capping in immature permanent teeth and to investigate potential factors (age, sex, tooth position, jaw, exposure site, capping material, temporary filling) contributing to the pulpal survival according to time. This study reveals survival rate (89.9%) after 1 year and (67.4%) after 3 years when MTA was used as direct capping agent. CH yielded (73.9%) and (52.5%) after 1 and 3 years respectively. Patients <40 years age group had better survival rate than older ones, and when exposure site was limited to occlusal site there was better survival than when it was on axial side. Other potential factors not significantly affect survival rates.
Partial Pulpotomy
Partial Pulpotomy is defined as "The surgical removal of coronal portion of vital as a means of preserving the vitality of the remaining coronal and ridiculer pulp tissues" [18].
Indications [37]
a. Restorable permanent teeth.
b. Deep Carious lesions or traumatic fractures longer than 24 hours with vital pulp exposure.
c. When pulpal inflammation is expected to be greater than normal and pulpal bleeding cannot be controlled within several minutes.
d. No symptoms of pulpits or with diagnosis of reversible pulpits.
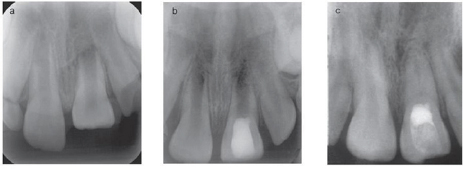
Technique (Figure 4)
The carious exposure generally occurs in the depth of a carious excavation. The cavity preparation presents convenient and effective location for the protection and seal of the pulp with pulp capping material. On the other hand, a traumatic fracture leaves the pulp exposed on the surface of the tooth and presents a problem in both retention of capping material and prevention of leakage. In addition to time of exposure, health of pulp before trauma, diameter of pulp exposure (1.5mm diameter is the critical), age of the tooth, no concomitant luxation injury and the stage of root development [73]. To address many of these challenges, Cvek procedure [74] was developed. The tooth is anaesthetized and isolated with rubber dam. A 1-2mm deep cavity is prepared into the pulp using sterile diamond bur of appropriate size at high speed with copious coolant. If bleeding is excessive the pulp is excavated deeper and cotton pellet moisture with 3-6% sodium hypochlorite for 10-20 second is placed over the exposed pulp. Sodium hydroxide serves as an excellent diagnostic tool to help to evaluate how far the inflammation has progressed into the pulp. A profuse bleeding that is difficult to stop indicates severe pulpal inflammation and the decision to proceed with pulpotomy [75].
Figure 4: Partial pulpotomy in upper central incisor.
a) Preoperative radiograph shows traumatic pulp exposure.
b) Partial pulpotomy capped with MTA.
c) Postoperative radiograph shows complete root formation.
Any blood clot should be removed. If homeostasis is achieved, hard setting calcium hydroxide is placed over pulpal tissue and sealed with permanent restoration [76] or MTA is mixed according to the manufacturer's instructions (3:1, powder to liquid), and placed directly over exposed pulp tissue and surrounding dentine with at least 1.5mm thickness [77]. Definitive restoration is either placed over MTA several days later or placed immediately after applying a layer flow able resin on unset MTA.
Mode of action
Similar to direct pulp capping, calcium hydroxide and mineral trioxide aggregate induce dentine bridge formation most likely by indirect mechanism through the release of growth factors from dentine matrix. Growth factors proteins stimulate the differentiation of post-natal stem cells in human dental pulp into odontoblasts-like cells that forms dentine bridge [59,78].
Treatment outcome
Studies have demonstrated that partial pulp capping of exposed permanent teeth with calcium hydroxide is an alternative treatment with good clinical outcome [79-81] and formation of reparative dentine [82]. Recently, mineral trioxide aggregate has been used successfully as pulp capping material after partial pulpotomy [8385]. In a prospective clinical study [86], partial pulpotomy was performed by grey MTA in 28 teeth with carious exposures. The patients were followed for 24 months. 79% of these teeth had normal response to vitality tests and with no radiographic evidence of pathosis.
In another prospective clinical and radiographic study [87], comparing CH and MTA as pulp capping materials after partial pulpotomy in cariously exposed teeth, it was reported that no significant differences in radiographic and clinical success rates between CH (91%) and MTA (93%). Hard tissue barrier was noticed under CH (55%) compared with (64%) under MTA. Long term evaluation of partial pulpotomy was (93.9%) after 49 months [88]. In a recent randomized clinical trial [50] comparing direct pulp capping and partial pulpotomy, showed no difference in pulp vitality at one year using CH. A recent systematic review (72) for vital pulp therapy in vital permanent teeth with cariously exposed pulp revealed success rate of partial pulpotomy was different depending on follow up time (>6 months -1 year, 97.6%), (>1-2 years, 97.5%), (>2-3 years, 97.6%) and (>3 years, 99.4%). Also showed that direct comparisons of weighed pooled success rate when using either CH or MTA as pulp capping medication revealed no statistically significant difference, while in indirect comparisons of weighted pooled success rate showed that CH was superior to MTA in partial pulpotomy.
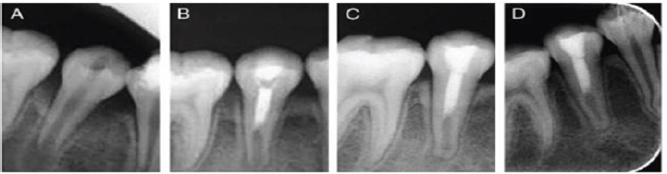
Full Pulpotomy
Full pulpotomy is defined as "The removal of entire coronal pulp to the level of the root canal orifice or as much as 2-3mm apical to the orifices" [18].
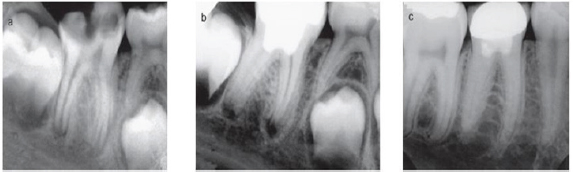
Figure 5: Pulpotomy in lower molar.
a) Preoperative radiograph with extensive decay and open apex.
b) After removal of caries and perform pulpotomy and resorted with amalgam.
c) Postoperative radiograph after periapical tissue healing and apex closure.
Indications [88]
a. Restorable permanent teeth.
b. Deep Carious lesions or traumatic fractures longer than 72 hours with vital pulp exposure.
c. When pulpal bleeding cannot be controlled within 10 minutes.
The main disadvantage of full pulpotomy is that pulp testing is not possible due to the loss of coronal pulp tissue, so radiographic follow up is extremely important.
Technique [88] (Figure 5)
Tooth is anaesthetized and isolated with rubber dam. The coronal pulp is removed to the level of root canal orifices. If there is bleeding, homeostasis is done by placing cotton pellet moisture with 3-6% sodium hypochlorite for 10-20 seconds. If bleeding cannot be controlled, then decision for lumpectomy should be taken. Any blood clot should be removed. If homeostasis is achieved, hard setting calcium hydroxide or MTA with bacterial tight seal and coronal restoration are carried out as with partial pulpotomy.
Mode of action
In response to pulpal dressing with calcium hydroxide or mineral trioxide aggregate apposition of hard tissue is achieved similarly as direct pulp capping and partial pulpotomy. Dentine bridge formation following apex genesis is a reparative process of dentine-pulp complex. However, the continued root formation is a normal physiological process [23].
Treatment outcome
In a case report [89], pulpotomy showed successful result in treatment of dens imagination in 2 mandible premolars. In histological study [90], comparing CH and MTA pulpotomy materials showed that teeth pulp-capped with MTA had significant less inflammation and more homogenous and continuous dentine bridge than those capped with CH. Another study [91] comparing CH and MTA as capping materials for 30 immature permanent teeth (15 with CH and 15 with MTA) and followed for 3, 6, 12 months showed that only 2 teeth that were capped with CH failed with association of pain and swelling. The authors recommend MTA as a suitable alternative to CH. Pulpotomy with MTA as pulp capping material was performed on symptomatic teeth with history of lingered pain [92] and histological observation showed complete dentin formation 2 months after treatment to all the treated teeth In another case series of pulpotomy with MTA as pulp capping material was performed on teeth with irreversible pulpitis [93] and followed for 19.7 months showed that only 1 of 19 teeth had persistence pain. Pulpotomy with CH as pulp capping material in symptomatic teeth [94] or with periodical involvement [90-92] showed complete resolution of symptoms, periodical healing and continued root formation. A recent systematic review [72] for vital pulp therapy in vital permanent teeth with curiously exposed pulp revealed success rate of full pulpotomy was different depending on follow up time (>6 months -1 year, 94%), (>1-2 years, 94.9%), (>2-3 years, 96.9%) and (>3 years, 99.3%). Also showed that direct and indirect comparisons of weighed pooled success rate when using either CH or MTA as pulp capping medication revealed that MTA was superior to CH in full pulpotomy.
Calcium Hydroxide Versus Mineral Trioxide Aggregate in Vital Pulp Therapies
The biological reactions resulting from application of bioactive materials to the pulp are confounded by several factors such as the formulation of applied material, concentration and rate of release of active ingredient, distance it has to diffuse in a chemically active form to the target cells, status of dentine, presence of infection and the health status of the pulp. It has been suggested that the type of biomaterial selected is of lesser consequence and that the quality of the seal of the cavity to prevent microbial ingress is the most important factor determining the success of the procedure [95].
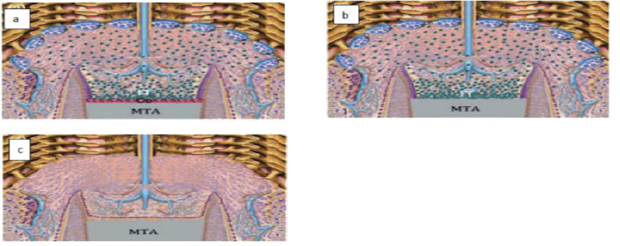
Hard tissue induced by calcium hydroxide (Figure 6)
When CH is placed against the pulp, the high PH of CH results in zone of liquefaction necrosis subjacent to CH and a deeper zone of coagulation necrosis next to vital pulp within the first week (approximately 1.5mm combined width incase of non-setting CH) [96]. Dentine matrix appeared around 30 days later once the cellular and vascular inflammatory events began to fade. Growth factor proteins, bone morphogenic proteins and bioactive molecules are released from dentin matrix and stimulate the differentiation of pulpal stem cells into odontoblasts-like cells [95]. Collagen is laid down approximating the necrotic zone and mineralized crystals are deposited in the region. Thickening of barrier and appearance of dentine-like tissue has been reported to be observed at around 4 weeks after pulp capping procedure and then dentin deposition begins [95].
Drawbacks of calcium hydroxide
Length of time for induction of coronal hard tissue that ranges from 2-3 months [96]. Non adhesive nature of the cement and its dissolution over time may lead to micro leakage and entry of bacteria to exposure site [97]. It is important to ensure seal by a restoration overlying the cement [71]. Bacterial contamination may also occur through tunnel defects (imperfection in dentine bridge due to inclusion of blood vessels) [64]. Impaction of particles of capping agent, enclosing action of CH up to 1.5mm compromising vascularity of the pulp and interference by blood clot are other factors responsible for failure of procedure Calcium hydroxide has limited solubility and infusibility, its antimicrobial effect is limited to superficial layers of pulp, thus CH may be successful only in cases of minimal inflammation. If inflammation is severe the procedure generally fails [94].
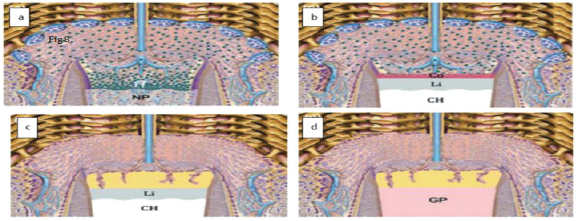
Figure 6: Calcium hydroxide and pulpal responses.
a) Exposed pulp, b) The high PH generates a zone liquefaction necrosis and a zone of coagulation necrosis, c) Sperical foci of calcification are formed that coalesce to form calcification zone and next to that collagen is formed, d) Bone like tissue is deposited containing cells and vascular inclusions.
Hard tissue induced by mineral trioxide aggregate (Figure 7)
When MTA is placed against the pulp, the high PH of MTA results in zone of coagulation necrosis next to vital pulp after one week. No zone of liquefaction necrosis is formed subjacent to MTA. High PH lasts for at least 8 weeks. Growth factor proteins, bone morphogenic proteins and bioactive molecules stimulate the differentiation of pulpal stem cells into odontoblasts-like cells which produce collagen matrix. A regular hard tissue barrier with cellar inclusion was seen after 2 weeks [95]. The hard tissue barrier appears to be formed earlier than under CH, with larger daily dentin increments, few vascular inclusions and with less inflammation [98]. MTA forms a very tight seal where it contacts the dentin walls, most likely due to a chemical bond between MTA and dentin; a layer of hydroxyapatite is created as a link [99]. This layer prevents and reduces bacterial penetration to the pulp; enhance biocompatibility, sealing ability and dentinogenic activity of MTA. This seal might be the main cause of successful performance of this material [100].
Drawbacks of mineral trioxide aggregate
Despite its many advantages, MTA has some drawbacks such as discoloration potential, presence of toxic elements in the material composition, difficult handling characteristics, long setting time, high material cost, an absence of a known solvent for this material and the difficulty of its removal after curing [101].
Internal bleaching was reported to be the treatment of discoloration due to MTA application in vital pulp therapy [102]. It should also be noted that the total amount of arsenic (that can cause toxicity) in all types of MTA is insignificant [103].
Figure 7: Mineral trioxide aggregate and pulpa! responses.
a) High PH generates a very narrow zone of coagulation necrosis,
b) Next to that zone reparative dentinogenesis zone is formed.
Apexification
Apexification is defined as "a method of inducing a calcified barrier in a root with open apex or the continued apical development of incompletely formed root in teeth with necrotic pulp" [18].
Indications [37]
a. Permanent teeth.
b. Non vital pulp with open apex and thin dentin walls.
c. Standard instrumentation technique cannot create apical stop.
Technique (Figure 8)
The tooth should be isolated with rubber dam. The first phase of treatment is to disinfect the root canal system to ensure periodical healing [22]. The canal length is estimated with a parallel preoperative radiograph, and after access to the canals is made, a file is placed to the determined length and the working length is confirmed by radiograph. Very light filling with copious irrigation using 0.5% sodium hypochlorite is performed. The canal is dried with paper points and creamy mix of calcium hydroxide is placed into the canal and sealed with temporary restoration for 1 to 4 weeks until there is no sign of infection [94].
Figure 8: Apeification with MTA as apical plug.
A) The canal is disinfected with copious irrigation.
B) Calcium phosphate is placed through the apex as a barrier.
C) 4mm of MTA is places at the apex.
D) The canal is filled with thermoplastized gutta-percha.
E) Bonded resin resorted is placed at level below CEJ.
Traditional apexification
Pure calcium hydroxide powder is mixed with sterile saline to a thick consistency. Ready-mixed commercial calcium hydroxide can also be used [104]. The calcium hydroxide is packed against the apical soft tissue with a plugged. This step is followed by backfilling with calcium hydroxide to completely fill the canal. The excess is removed from access cavity to level of the root orifices, and a well-sealing temporary restoration. A radiograph is taken in order to evaluate formation of hard tissue barrier and washout of CH [94]. Controversy exists as to whether or how often the calcium hydroxide should be changed. Several studies [105-107] suggested placing the paste only once and waiting for radiographic evidence of barrier formation, and that there was nothing gained by repeated root filling either monthly or after 3 months. A recent study [108] reported that apical barrier was formed in short time (30 weeks) due to the minimal disturbance of apical area by avoiding repeated instrumentation and dressing changing. On the other hand, a number of studies [109-112] suggested that regular replacement of dressing allows clinical assessment of barrier formation and may increase the speed of bridge formation. This is due to the solubility of CH when comes in contact with tissue fluids [25]. Other investigators [113,114] suggested that calcium hydroxide should be replaced only when symptoms develop or the material dissolves out of the canal when assessed radio graphically. However, the size of foramen opening and stage of development may be important factors to determine the time to replace the dressing [115]. Radiograph may not be a relevant to evaluate calcium hydroxide washout; because the additives (barium sulphate) that are responsible for CH radio-opacity do not washout as readily as calcium hydroxide so that if they are present in the canal, evaluation of washout is not possible [94]. When hard tissue barrier is indicated on radiograph, the calcium hydroxide is washed out with sodium hypochlorite and apical barrier is detected gently by a file or a paper point. The entire canal is then filled with thermo plasticized gutter perch [94].
One step/visit apexification (Figure 8)
MTA can be used as apical plug or even as a root canal obscuration [116]. If MTA will be used, calcium sulphate is pushed through the apex to provide a restorable extra ridiculer matrix against which to pack the MTA [117]. Other types of matrix were also reported as collagen matrix [118]. However, MTA can be placed without matrix and care must be taken as MTA should be confined to root canal end [119]. MTA should be mixed according to manufacturer's instructions to a thick creamy consistency and placed 1-1.5 mm short of working length using a carrier and condensed with minimal pressure. This is repeated until approximately 5mm of material is deposited apically [119]. Moist cotton is placed in the canal for at least 6 hours and the entire canal is filled with thermo plasticized gutter perch [120] or the root filling is placed immediately over MTA because the tissue fluid of the open apex will probably provide enough moisture for MTA to set [121].
Soften filling technique is indicated in these canals because the apical diameter is larger than the coronal diameter. Care must be taken to avoid excessive lateral forces during obscuration technique [94]. It was reported that short term canal medication with calcium hydroxide followed by an apical plug of MTA may increase the marginal adaptation of MTA and increase long-term prognosis for teeth with necrotic pulps and open apices [122,123]. Because the acidic environment due to inflammation can cause porosity in set MTA and affect its sealing ability [124,125].
Mode of action
In apexification procedures, the infected necrotic is removed up to the apex by means of mechanical debridement and antiseptic chemical irrigation [23]. Accordingly it is unlikely that reparative dentine can be formed because of the absence of pulp. The calcified barrier at open apex has been described as cemented-like tissue and asteroid-like tissue [126].
Calcium hydroxide and mineral trioxide aggregate stimulate release of growth factors and bioactive molecules from cemented matrix and signal progenitor/stem cells in periodontal ligament to differentiate into cement oblast-like cells that forms cemented tissue [127].
CH and MTA also stimulate release of growth factors and bioactive molecules from alveolar bone marrow matrix and signal progenitor/stem cells in periodontal ligament and mesenchymal stem cells in alveolar bone marrow to differentiate into osteoblastic- like cells that forms asteroid tissue [128].
It was shown that MTA offers a biological substrate for cement oblasts attachment and growth as well as production of mineralized matrix gene (osteocalcin) and protein expression over MTA [36]. Continued physiological root development of immature permanent tooth with infected necrotic pulps and apical periodontitis after apexification procedure treated with calcium hydroxide was reported [24,129]. The newly formed root apical to the hard tissue barrier had normal root structure with pulp tissue in the canal space surrounded by dentine and cemented. It appears in this case that apical papilla and her twig's epithelial root sheath (HERS) have survived despite apical periodontitis. HERS cells are capable of signaling stem cells in apical papilla to differentiate into odontoblasts and produce root dentine [129].
Treatment outcome
In a clinical study [127], apexification performed with calcium hydroxide in 34 teeth revealed that only 4 teeth failed to form hard tissue barrier. In a large retrospective clinical study [128], 328 located non-vital incisors were treated with CH and followed for 4 years. This study showed periodical healing in 95% of teeth and increased frequency of fracture from 77% in least developed teeth to 28% in most developed roots. Two large clinical studies [129,130] involved treatment with CH and subsequent guttapercha filling have shown average healing rate 95%. In a review of ten studies [111], the use of CH for apical barrier formation was successful in 74-100% of cases irrespective of the proprietary brand used. Continued physiological root development of immature permanent tooth with infected necrotic pulps and apical periodontitis after apexification procedure treated with calcium hydroxide was reported [24,129]. The newly formed root apical to the hard tissue barrier had normal root structure with pulp tissue in the canal space surrounded by dentine and cemented. In another retrospective study [130], 23 necrotic immature teeth treated for either long term with CH (n=16) or short term with MTA (n=7). All necrotic immature teeth achieved a nearly normal root development after (mean 16 months). In a case series study [131], a successful outcome in 10 of 11 teeth with necrotic pulps and open apices after application of MTA was observed and apical barrier formation after 24 months. Two studies [132,121] of necrotic pulps and open apices treated either with white MTA or grey MTA as apical barriers, radiographic examination revealed 81% success for these cases.
In another case series study [133], used MTA as apical plug in 17 incisors and followed for 12.53 months showed that 94.1% were assessed as being successful clinically, whereas 76.5% were reported to be successful radio graphically. In a large retrospective clinical investigation [134] on teeth with necrotic pulps and open apices that were treated with MTA as apical plug comparing 1 visit with 2 visits procedures, showed no significant difference between the two treatment protocols. A recent clinical and radiographic assessment [135] of two types of White MTA used as apical plug after initial dressing with CH showed overall success rate 95.5% after 23.4 months follow up.
Another recent cohort study [136] assessing outcome of treatment of open apices with orthograde placement of MTA apical plug, reported that teeth with and without preoperative periodical radiolucencies showed healed rates of 85% and 96% respectively after 4 years follow up. In a comparative study [137] on permanent maxillary incisors with CH or MTA, demonstrated that mean time for CH (7 months) to form hard tissue barrier is significantly longer than time needed by MTA (3 months) to induce similar barrier. Another investigation [138] compared MTA with CH for treatment of teeth with immature roots and observed for 12 months. None of MTA cases showed signs of clinical and radiographic failure, whereas 2 of 15 teeth in CH group had tenderness to percussion and persistent periodical inflammation.
In a report of three cases [137] describing treatment outcome of unintended extrusion of MTA into periodical tissue during apical barrier treatment showed that only in one case after 4 years follow up MTA had resorted and periodical lesion had healed while in two other cases the patient remained symptomatic and scheduled for periodical surgery. Another two studies [138,139] revealed that complete periodical healing is possible despite extrusion of MTA periodically.
A meta-analysis [139] of 30 article published in recent years indicated that MTA has high clinical success rate, provides best seal, shows superior biocompatibility, and the only root end filling when compared with amalgam, IRM, super EBA. A recent systematic review and meta analysis [140] comparing MTA and CH as apical barriers used in apexification of immature permanent teeth showed that regarding clinical success and apical barrier formation, either CH or MTA may be used for apexification of immature teeth.
Calcium Hydroxide Versus Mineral Trioxide Aggregate in Non-Vital Pulp Therapies
Apexification technique first requires elimination of bacterial infection from root canal and prevention of re-infection of this space [22]. Disinfection of the root canal space is straight forward for most cases; however, there is no natural apical construction or stop against which a suitable root filling material can be placed to prevent re-infection of this space [20]. Therefore one of the aims of treatment is to produce apical barrier against which a root canal filling can be placed and prevent extrusion of material into surrounding tissue. Studies vary in assessment of time required for apical formation in apexification using calcium hydroxide. In a review of ten studies [111], reported an average for apical barrier formation ranging from 5-20 months. In another study [118] of 44 non-vital teeth undergoing CH apexification, found that the mean time to barrier formation was ranging from 4-16 months. The strongest predictor of rapid barrier formation was the rate of change of calcium hydroxide and also the narrower initial apical width the faster apical barrier formation.
It was reported that the infection or the presence of periodical radiolucency at the start of treatment increases the time required for barrier formation [141]. However, other studies [130,142] refuted this finding and stated that there is no relation between preoperative infection or radiolucency and rate of formation of apical barrier. It was found [143] that in the presence of symptoms, the time required for apical closure was extended by approximately 5 months to an average of 15.9 months.
comparative study [137] on permanent maxillary incisors with CH or MTA, demonstrated that mean time for CH (7 months) to form hard tissue barrier is significantly longer than time needed by MTA (3 months) to induce similar barrier.
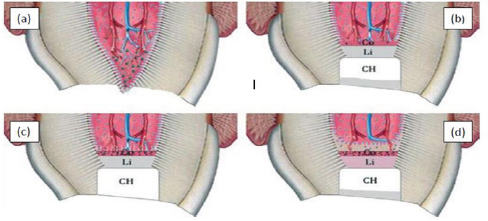
Figure 9: Calcium hydroxide and periapical changes.
a) Infected pulp and inflammation.
b) The high PH generates a zone liquefaction necrosis and a zone of coagulation necrosis.
c) New hard tissue forming cells from periapical tissue and formation of cementoid and osetoid tissue.
d) Root filling with gutta-percha, notice vascular inclusion within hard apical barrier.
Hard tissue induced by calcium hydroxide (Figure 9)
When CH is placed against periodical tissues, the high PH value of CH results in zone of liquefaction necrosis subjacent to CH and a deeper zone of coagulation necrosis next to periodical tissues [103]. This coagulation necrosis zone stimulates release of growth factors (wound healing signals) and bioactive molecules from cemented matrix and alveolar bone marrow matrix. The response to these factors appear to be recruitment of new hard tissue forming from apical tissue, these are usually of cementoblastic but may also be osteoblastic origin [144]. During this process numerous vascular inclusions may occur [144].
Drawbacks of calcium hydroxide
Length of time for induction of apical hard that ranges from 6-18 months [111,118]. Such lengths of time may delay completion of treatment and failure to comply the patient. Incomplete apical hard tissue barrier occurs due to vascular inclusions and may allow bacterial invasion through these defects [144].
Long term calcium hydroxide dressing cause changes in physical structure of dentine [145,146]. Such changes may weaken the dentine and lead to root fractures [128,147,148]. However, in a recent systematic review [149] of the effect of non-setting calcium hydroxide and mechanical properties of dentine showed that there are no clinical trials examining the relation between calcium hydroxide intra canal dressing and root fracture. However, the majority of in vitro studies showed reduction in mechanical properties of ridiculer dentine after exposure to CH for 5 weeks or longer.
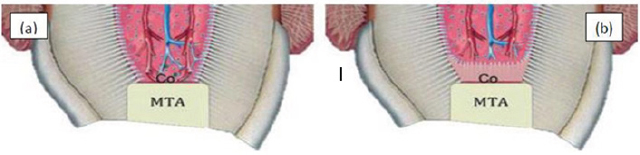
Hard tissue induced by mineral trioxide aggregate (Figure 10)
When MTA is placed against periodical tissues, the high PH value of MTA results in very narrow zone of coagulation necrosis next to periodical tissues [103]. MTA modulates cytokines production and encourages differentiation and migration of hard tissue producing cells, whereby hydroxyapatite is formed on MTA surface and biological seal is created [108].
Figure 10: Mineral trioxide aggregate and pulpal responses.
a) MTA induce release of wound healing signals.
b) High PH generates a very narrow zone of coagulation necrosis.
c) Next to that zone reparative dentinogenesis zone is formed.
Drawbacks of mineral trioxide aggregate
MTA has some drawbacks such as difficult handling characteristics, long setting time [108]. The porosity in set MTA has been identified as potential drawback for the material. It can allow potential penetration of bacteria or by-products. However, studies [150,151] have shown after MTA placement, a layer of hydroxyapatite forms over the material that fills the voids and surface defects.
Reinforcement of Thin Dentinal Walls
Root strengthening interventions can be divided into the following four categories [152]. Adhesive resins as root strengthening material the use of adhesive sealers in the root canal system might possibly enhance resistance to fracture of thin weak dentinal walls as in immature permanent teeth [153].
Glass monomer cements as root strengthening material
Glass monomer cements have been shown to have long term adhesion effects by bonding to the hydroxyapatite component of dentine [154].
Intra-canal composite resin as strengthening root material
The use of newer dentine bonding techniques has been shown to strengthen the thin dentine walls of immature teeth [155]. Greater resistance to root fracture after placement of 4mm thick apical plug of MTA followed by intra-canal composite resin when compared with MTA followed by gutta-percha and sealer was reported [156]. Before placement of composite, the internal dentine walls of canal are acid etched and dentine bonding agent is applied to the walls. 2mm increment of condensable light cure composite resin is placed in the canal and cured. This step is repeated until the canal and access opening are obliterated [157]. However, due to incomplete polymerization in deeper depths of the canal because of limited transmission of light through the material, clear plastic posts have been developed to allow light transmission throughout the canal and cure entire mass of composite [158]. Resin modified glass monomer with translucent curing post showed significant increased resistance to root fracture when compared with gutter -perch alone in open apex [159].
Fiber glass posts
Fiber-reinforced composite posts have been reported to have biomechanical properties close to those of dentine [160] and similar modulus of elasticity to that of dentine [161]. This seems to reduce stress transmission to the root canal walls by the post and thus avoiding possible root fracture [162,163].
Revitalization treatment
Revitalization is a new treatment procedure that may replace apexification procedures. Is defined as "The attempts to revive tissues in the pulp space and continue root formation in immature teeth with non-vital pulp" [164]. In this treatment, the ideal goal is to prepare an appropriate environment inside the root canal space (i.e., absence of bacteria and necrotic pulp tissue, presence of a scaffold and a tight coronal seal) that promotes repopulation of these stem cells, regeneration of pulp tissue, and continuation of root development [165].
Indications and recommendations [166]
The traumatized tooth must be non-vital and not be suitable for apex genesis, apexification, partial pulpotomy, or root canal obscuration treatments. The tooth must be permanent and immature with open apex that is wide to a diameter of 1.1mm or larger and exposed pulp. The patient must be aged 7-16 years, in good health and have parent's guardians willing to take them to attend multiple appointments. Antibiotic paste can be used as additional disinfectant to sodium hydroxide and the patient needs to be warned about the potential for discoloration. An anesthetic without a vasoconstrictor should be used when attempting to induce bleeding into the root canal. A thin layer of white MTA or calcium hydroxide should be placed over the blood clot. An endodontic sealer is not compatible and cannot be used. The tooth should be restored with resin modified glass monomer to help prevent micro leakage.
Technique (Figure 11)
Several case reports and case series have recently introduced detailed regenerative endodontic techniques applied to cases of necrotic immature permanent teeth [167-170]. The tooth is anesthetized and isolated with rubber dam. The canal is disinfected without mechanical instrumentation but with copious irrigation with 2.5% sodium hypochlorite. After drying the canal, tri-antibiotic paste, sometimes called Hoshino's paste [171]. The paste contains 200mg ciprofloxacin, 500mg metronidazole, and 100mg minocycline. The triple antibiotic paste is placed in contact with necrotic pulp inside the root canal for up to 1 month before revascularization procedure. After one month, antibiotic is removed from canal by irrigation with 2.5% sodium hypochlorite and 17% ethylene diamine-tetracetic acid. Conditioning the dentin surface with ethylene diaminetetra acetic acid may enhance the adherence and differentiation of dental pulp stem cells during pulp regeneration [172,173].
Figure 11: Revitalization procedure.
a) Preoperative radiograph with periapical lesion and open apex.
b) After 3 months elongation with periapical lesion and open apex.
c) After 8 months shows narrowing if apex and thickening of dentine walls.
d) After 18 months shows healing of lesion and apical closure.
Bleeding is induced till the level of canal orifice by passing a file beyond working length and causing irritation in periodical tissue. The bleeding is left for 15 minutes so that the blood would clot. Blood clot serves as a protein scaffold and permitting threedimensional in growth of tissue. MTA is placed over blood clot followed by moist cotton and well-sealed temporary restoration. After few days the cotton is removed and bonded restoration is placed over set MTA.
Patient should be recalled every 6 month for clinical and radiographic examination to ensure thickening of dentine walls and continued root formation. Staining of the dentin by minocycline, a derivative of tetracycline, has been reported. Therefore, some authors suggest eliminating the minocycline and keeping only metronidazole and ciprofloxacin in the antibiotic paste (binate) [174]. While others suggest that sealing dentinal tubules with dentine bonding agent may reduce the intensity of discoloration but did not prevent it [175-177].
Calcium hydroxide paste is another intracranial medicament that has been used to disinfect the canal before inducing bleeding [178,179]. However CH may not be suitable if there is remaining vital pulp tissue in the canal. The direct contact of CH paste with tissue will induce formation of layer of calcified tissue which may occlude the pulp space [180]. Another concern is that CH may damage her twig's epithelial root sheath and thereby destroy its ability to induce the nearby undifferentiated cells into odontoblasts [181].
Another technique was reported [181,182], without inducing intracranial bleeding and all cases showed noticeable apical maturation with increased root length but a significant narrowing of canal space.
Mode of action
The precise nature of hard tissue formed inside the canal space and continued root development of immature permanent teeth with apical periodontitis/abscess following revascularization procedures in humans is not known, because no histological studies are available. It has been assumed that periodontal ligament tissue might grow into the canal space and deposit cemented on the canal walls after revascularization procedures [181]. HERS cells play an essential role in root development. If HERS cells survived in apical periodontitis/abscess after revascularization procedures, they can signal progenitor/stem cells in the periodontal ligament to differentiate into cement oblast-like cells and produce cemented- like tissue to promote root development [143]. If the apical papilla also survived in apical periodontitis/abscess after revascularization procedures, HERS cells will signal apical papilla cells to differentiate into root primary odontoblasts and promote root dentine formation [183]. Thus, the incompletely formed root will continue its normal physiological development after revascularization procedures.
However, no pulp-like tissue or dentine formation was present in the apical portion of the canal space of immature teeth with apical periodontitis/abscess after revascularization procedures in animal studies [184]. This indicates that the apical papilla did not survive. Continued root development was evidenced by apical deposition of cemented without dentin after revascularization procedures in animal studies, probably because HERS cells survived in apical periodontitis [185].
When the canal dentine was treated with 17% ethylene diaminetetra acetic acid (EDTA) to expose dentine matrix, dentin- associated mineralized tissue appeared to be tightly attached to the canal dentine walls, In addition, EDTA may also cause the release of bioactive growth factors from dentine matrix [183]. If the tissues present inside the canal space in human immature permanent teeth with apical periodontitis/abscess after revascularization procedures are similar to that observed in animals, then revascularization is a reparative process with the loss of pulp biological function. Cemented and asteroid tissues are not normally present as part of the pulp tissue. These tissues inside the canal space may function like periodontal tissues [186]. Histological studies are necessary to verify whether repair or regeneration of the pulp tissue occurs inside the root canal space following revascularization procedures in humans.
In immature permanent teeth with a long-standing apical periodontitis/abscess in adult patients, the apical papillae and HERS cells may be severely damaged, thus hindering the potential of continued root development. Furthermore, the functionality of stem cells is age-related decline owing to change in the intrinsic factors of stem cells and diminished signals within the extrinsic local and systemic environment that modulate the function of stem cells or their progeny [23].
The incompletely developed roots of immature permanent teeth with infected necrotic pulp and apical periodontitis are not attributable to the destruction of previously formed roots but are attributable to the inhibition or damage of HERS cells. Therefore, continued root development of human immature permanent teeth with infected necrotic pulp and apical periodontitis/abscess after revascularization procedures does not appear to be a regenerative process. It could be considered a physiological process if both apical papilla and HERS survive in apical periodontitis/abscess after periodical wound healing [23]. Regeneration of the dentine-pulp complex after complete pulp necrosis or irreversible pulpitis with apical periodontitis is likely to occur only by tissue engineering [187] or gene therapy [188].
Treatment outcome
Most of evidence for revascularization procedures involves successful case reports and case series [178,180,181]. The use of triple antibiotic paste was reported [182] to be effective for disinfection of infected root canals, setting the conditions for subsequent revascularization and showed healing of sinus tract [189]. A few cases have reported positive response to cold/EPT after revascularization procedures [179,184,189,190]. However it was found that both the coronal level of regenerated tissue and the thickness of filling materials placed over this tissue affect the presence or absence of responses to EPT and cold. The authors reported positive responses to both EPT and cold in an immature maxillary premolar with a coronal MTA plug placed close to the cement enamel level [190]. Cases treated with calcium hydroxide paste as intracranial medicament to disinfect the canal before inducing bleeding showed continued root development and thickening of dentin walls [188,189].
In a recent case report [191] of revascularization treatment outcome of 2 immature necrotic mandible first molars with symptomatic apical periodontitis and chronic apical abscess, respectively. In both teeth, the root canals received 3-week disinfection with the triple antibiotic paste. After 18 and 15 months (cases 1 and 2, respectively), the teeth showed advanced root development, thickening of the root walls, and complete periradicular healing. In a recent study [192] reporting long term outcome of three cases with necrotic immature teeth and followed for 24, 48 and 42 months showed that all of the revascularized teeth showed satisfactory clinical and radio graphical performances at long-term follow-ups. In well-selected cases, the revascularization technique may be a trustworthy alternative to conventional apex genesis or apexification. A recent study [193] examining the effect of different irritant on the survival of human stem cells of the apical papilla showed that the irrigation protocols evaluated in this study that contained EDTA promoted SCAP survival, whereas protocols in which 2% CHX was used appeared cytotoxic with no viable cells.
Also showed that chelating effect of EDTA promotes the release of dentin-derived growth factors that were previously embedded into dentin during the process of dentin genesis. These growth factors have been shown to foster proliferation, survival, and differentiation of dental stem cells. A recent in vitro study [194] examining the effect of medications used in regeneration techniques on chemical structure of immature human ridiculer dentine showed that long-term exposure of ridiculer dentin to Ca(OH)2 or antibiotic pastes might negatively affect the mechanical properties of dentin and increase the susceptibility to root fracture because of either collagen degradation in the case of Ca(OH)2 or excessive demineralization in the case of antibiotic pastes.
The null hypothesis that the 3 intracranial medicaments (triantibiotic, biantibiotic pastes and calcium hydroxide) have no significant effect on the chemical structure of reticular dentin was rejected. A retrospective evaluation [195] of radiographic outcomes discovered that regenerative endodontic treatment with triple antibiotic dressing increased root wall thickness significantly more than either calcium hydroxide or form cresol. In addition, this study revealed that in cases disinfected with calcium hydroxide, the radiographic location of calcium hydroxide inside the root canal space influenced the root development. When calcium hydroxide was radio graphically limited to coronal half of the root canal space, the increase in root wall thickness was greater than when it was placed beyond coronal half.
Drawbacks of revitalization
There are several drawbacks and unfavorable outcomes of regeneration procedure [196].
Discoloration
A study [185] demonstrated that the main reason for tooth discoloration after treatment was the contact of minocycline in the triple antibiotic paste with coronal dentinal walls during treatment procedure. A recent study [187] suggested sealing dentinal walls of the access cavity by using dentin bonding agent and composite resin before placement of triple antibiotic paste inside the canal. A practical way to prevent discoloration is replacing minocycline with an antibiotic that does not stain teeth. It was reported [197] that successful regenerative endodontic treatment of a maxillary central incisor by using cofactor instead of minocycline in the antibiotic mixture. Another two studies [192,198] introduced novel method to shorten the treatment period and also prevent tooth discoloration by omitting the intracranial medication process.
Treatment period
The required time for disinfection of the root canal space with triple antibiotic paste or calcium hydroxide and increased number of clinical sessions (compared with one-visit MTA apical barrier technique) are other drawbacks of regenerative endodontic treatment. The time spent on antibiotic disinfection in clinical studies varies between one [199] to eleven weeks [200].
Poor root development
Ideal root development pattern in immature teeth includes increase in root length, increase in root wall thickness, and formation of the root apex. In some studies, the outcome of regenerative endodontic treatments of necrotic immature teeth was lower than ideal, including absence of increase in root length [184,201], absence of increase in root wall thickness [184,202], or lack of formation of tooth apex [202]. Formation of a hard-tissue barrier inside the canal between the coronal MTA plug and the root apex is another reported unfavorable outcome [202].
A recent study [202] revealed that root development potential of immature necrotic teeth is related to the vitality of her twig epithelial root sheath. Therefore, there might be a correlation between dental history and quality of root development; the longer the duration of pulp necrosis, the lower the quality of root development after regenerative endodontic treatments.
Insufficient bleeding
A recent study [203] revealed that mesenchymal stem cells are delivered into root canal space after bleeding induction in human teeth, a phenomenon that did not happen in the absence of blood clot inside the disinfected root canal space. An animal study [204] demonstrated that root canals that had a blood clot formation inside them after disinfection had better radiographic outcome compared with those without blood clot. To facilitate bleeding after root canal disinfection, using local anesthetics without vasoconstrictors is recommended [184]. However, lack of bleeding or insufficient bleeding after using plain local anesthetics is reported [184,205]. On the other hand, several cases with sufficient bleeding and lack of root development have been reported [184,206,207]. In addition, there are several reports [174,191,192] of successful regenerative endodontic treatment and continued root development without bleeding induction.
Root canal calcification
Root canal calcification/obliteration is another problem after regenerative endodontic treatment of necrotic immature teeth that is reported in cases disinfected by calcium hydroxide [208212]. On the other hand, there is no report of complete root canal calcification/ obliteration in cases disinfected by triple antibiotic paste.
References
- Pashley DH, Liewehr FR, Cohen S, Burns RC (2006) Structure and function of dentin-pulp complex. Pathway of the pulp, (9th edn), St Louis, Mosby, USA, p. 465.
- Holland GR, Trowbridge HO, Rafter M, Torbinejad M, Walton RE, et al.(2009) Protecting the pulp, preserving the apex. Endodontics, principles and practice, (4th edn), Philadelphia, USA, pp. 26-34.
- Kim S, Trowbridge H, Suda H, Cohen S, Burns RC, et al. (2002) Pulpal reactions to caries and dental procedures. Pathway of the pulp, (8th edn), St Louis, Mosby, USA, p. 573.
- Reeves R, Stanley HR (1966) The relationship of bacterial penetration and pulpal pathosis in carious tooth. Oral Surg Oral Med Oral Pathol 22(1): 59-65.
- Thomas MS, Kundabala M (2012) Pulp hyperthermia during tooth preparation, the effect of rotary instrument, lasers, ultrasonic device and airborne particles abrasion. J Calif Dent Assoc 40(9): 720-731.
- Majör IA, Ferrari M (2002) Pulp-dentin biology in restorative dentistry. Part 6: Reactions to restorative material, tooth-restoration interface and adhesive techniques. Quintessence Int 33(1): 35-63.
- Robertson A, Andreasen FM, Andreasen JO, Noren JG (2000) Long term prognosis of crown-fractured permanent incisors. The effect of stage of root development and associated luxation injuries. Int paediatr Dent 10(3): 191-199.
- Rosenberg PA, Schindler WG, Krell KV, Hicks ML, Davis SB, et al. (2009) Identify the endodontic treatment modalities. J Endod 35(12): 16751694.
- Brandt K, Kortegaard U, Poulsen S (1988) Longitudinal study of electrometric sensitivity of young permanent incisors. Scand J Dent Res 96(4): 334-338.
- Pileggi R, Dumsha TC, Myslinksi NR (1996) The reliability of electric pulp tests after concussion injury. Endodon Dent Traumatol 12(1): 1619.
- Jafarzadeh H (2009) Lasser Doppler floemetry in endodontics. A review Int Endod J 42(6): 476-490.
- Setzer FC, Kataoka SH, Natrielli F, Gondim-junior E, Caldeira CL, et al. (2012) Clinical diagnosis of pulp inflammation based on pulp oxygenation rates measured by pulse oximetry. J Endod 38(7): 880-883.
- Patel S (2009) New dimension in endodontic imaging: Part 2. Cone beam computed tomography. Int Endod J 42(6): 462-475.
- Souza RA, Gomes SC, Dantas Jda C, Silva-Sousa YT, Pecora JD, et al. (2004) Importance Of diagnosis in the pulpotomy of immature permanent teeth. Braz Dent J 18(3): 244-247.
- Camp JH (2008) Diagnosis dilemmas in vital pulp therapy: treatment for the toothache is changing, especially in young immature teeth. Pediatr Dent 30(3): 197-205.
- Mejare IA, Axelsson S, Davidson T, Frish F, Hakeberg M, et al. (2012) Diagnosis of the condition of the dental pulp, A systematic review. Int Endod J 45(7): 597-613.
- Abu-Tahun I, Torabinejad (2012) Management of teeth with vital pulps and open apices. Endo Topics 23: 79-104.
- Panuroot A, Pairoj L (2011) Vital pulp therapy in vital permanent teeth with cariously exposed pulp, a systematic review. J Endod 37(5): 581587.
- Glickman GN, Bakland LK, Fouad AF, Hargreaves KM, Schwatrz SA, et al. (2009) Diagnostic terminology, report of an online survey. J Endod 35(12): 1625-1633.
- Cho SY, Seo DG, Lee SJ, Lee J, Lee SJ, et al. (2013) Prognostic factors for clinical outcome according to time after pulp direct pulp capping: J Endod 39(3): 327-331.
- Bakland LK, Andreasen JO, Andreasen FM, Andreasen L (2007) New Endodontic procedures using mineral trioxide aggregate for teeth with traumatic injuries. Textbook and color Atlas of traumatic injuries to teeth. (4th edn), Ames, Lowa. Blackwell Munksgaard, USA, pp. 658-668.
- Mass E, Zilberman U (2011) Long term radiographic pulp evaluation after partial pulpotomy in young permanent molars. Quintessence Int 42(7): 547-554.
- Teixeira LS, Demarco FF, Coppola MC, Bonow ML (2001) Clinical and radiographic evaluation of pulpotomies performed under intrapulpal injection of anesthetic solution. Int Endod J 34(6): 440-446.
- Caliskan MK (1993) Success of pulpotomy in the management of hyperplastic pulpitis. Int Endod J 26(2): 142-148.
- Torebinajad M, Chivian N (1999) Clinical applications of mineral trioxide aggregate. J Endod 25(3): 197-205.
- Trowbridge HO, Hargreaves KM, Goodis HF (2002) Histology of pulpal inflammation. Seltzer and Bender's dental pulp. (3rd edn), Coral stream, IL. Quintesense Publishing Co Inc. USA, pp. 227-245.
- Caliskan MK (1995) Pulpotomy of carious vital teeth with periapical involvement. Int Endod J 28(3): 172-176.
- (2003) American Association of Endodontists, Glossary of endodontic terms, (7th edn), Chicago, USA.
- Webber RT (1984) Apexogenesis versus apexification. Dent Clin N Am 28(4): 669-697.
- Shah N, Logani A, Bhaskar U (2008) Efficacy of revascularization to induce apexification/apexogenesis in infected, nonvital, immature teeth: A Pilot clinical study. J Endod 34(8): 919-925.
- Morse DR, Larnie J, Yesilsoy C (1990) Apexification, review of the literature. Quintessence Int 21(7): 589-596.
- Rafter M (2005) Apexification: a review. Dent Traumatol 21(1): 1-8.
- Lin LM, Rosenberg PA (2011) Repair and regeneration in endodontics. Int Endod J 44(10): 889-906.
- Fatma B, Nekoofar MH, Gungy M, Paul M (2013) The effect of various mixing and placement techniques on compressive strength of mineral trioxide aggregate. J Endod 39(1): 111-114.
- Yang SF, Yang ZP, Change KW (1990) Continuing root formation following apexification treatment. Dent Traumatology 6(5): 232-235.
- Mohammadi Z, Dumme PMH (2011) Properties and applications of calcium hydroxide in endodontics and dental traumatology. Int Endod J 44(8): 697-730.
- Siqueira JF, Lopes HP (1999) Mechanism of antimicrobial activity of calcium hydroxide, a critical review. Int Endod J 32(5): 361-369.
- Estrela C, Sydney GB, Bammann LL, Felippe O (1995) Mechanism of action of calcium and hydroxyl ions of calcium hydroxide on tissue and bacteria. Braz Dent J 6(2): 85-90.
- Pisanti S, Sciaky I (1964) Origin of calcium in the repair wall after pulp exposure in the dog. J Dent Res 43: 641-644.
- Farhad A, Mohammadi Z (2005) Calcium hydroxide, a review. Int Dent J 55(5): 293-301.
- Kratchman SI (2004) Perforation repair and one step apexification procedure. Dent Clin N Am 48(1): 291-307.
- Camilleri J, Montesin FE, Brady K, Sweeney R, Curtis RV, et al. (2005) The constitution of mineral trioxide aggregate. Dent Mater 21(4): 297-303.
- Torabinejad M, Watson TF, Pitt Ford TR (1993) Sealing ability of a mineral trioxide aggregate when used as a root end filling material. J Endod 19(12): 591-595.
- Torabinejad M, Hong CU, McDonald F, Pitt Ford TR (1995) Physical and chemical properties of a new filling material. J Endod 21(7): 349-353.
- Fridland M, Rosado R (2003) MTA solubility and porosity with different water to powder ratios. J Endod 29(12): 814-817.
- Torabinejad M, Masoud P (2010) Mineral trioxide aggregate, a comprehensive review, Part I: Chemical, physical and antibacterial properties. J Endod 36(1): 16-27.
- Maedi H, Nakano T, Tomokiyo A, Fujii S, Wada N, et al. (2010) Mineral trioxide aggregate induces bone morphogenetic protein-2 expression and calcification in human periodontal ligament cells. J Endod 36(4): 647-652.
- (2009) American Academy of Pediatric Dentistry Clinical Affairs Committee, Pulp Therapy Subcommittee: Guideline on pulp therapy for primary and immature permanent teeth. Pediatr Dent 31: 179-186.
- Bogen G, Chandler NP (2012) Pulp preservation in immature permanent teeth. Endod Topic 23: 131-152.
- Bjørndal L, Larsen T, Thylstrup A (1997) A clinical and microbiological study of deep caries lesions during stepwise excavation using long treatment intervals. Caries Res 31(6): 411-417.
- Mertz-Fairhurst EJ, Curtis JR, Ergle JW, Reuggeberg FA, Adair S, et al. (1998) Ultraconservative and cariostatic sealed restoration, results at year 10. J Am Dent Ass 129(1): 55-66.
- James L, Paul E (2011) Problem solving in endodontics: Problem solving challenges in dentin hypersensitivity and vital pulp therapy, (5th edn), Elsevier, Mosby, USA, p. 139.
- Reeves S, Stanley HR (1966) The relationship of bacterial penetration and pulpal pathosis in caries teeth. Oral Surg Oral Med Oral Path 22: 5260.
- Tziafas D (2004) The future role of molecular approach to pulp-dentinal regeneration. Caries Res 38(3): 314-320.
- Smith AJ, Cassidy N, Perry H (1995) Reactionary dentinogenesis. Int J Develo Bio 39(1): 273-280.
- Gruythuysen RJ, van Strijp AJ, Wu MK (2010) Long term survival of in direct pulp treatment performed in primary and permanent teeth with clinically diagnosed deep caries lesion. J Endod 36(9): 1490-1493.
- Bjørndal L (2008) Indirect pulp therapy and stepwise excavation. J Endod 34(7): S29-S33.
- Thomson V, Craig RG, Curro FA (2008) Treatment of deep caries lesions by complete excavation or partial removal, a critical review. J Am Dent Ass 139(6): 705-712.
- Miyashita H, Worthington HV, Qualtrough A (2007) Pulp management for caries in adults, maintaining pulp vitality. Cochrane Database Sys Rev (2) : CDOO4484.
- Ricketts DNJ, Kidd EAM, Innes N (2006) Complete or ultraconservative removal of decayed tissue in unfilled teeth. Cochrane Database Sys Rev(3) : CDOO3808.
- Bjørndal L, Reit C, Bruun G, Markvart M, Kjaeldguaard M, et al. (2010) Treatment of deep caries lesion in adults, randomized clinical trial comparing stepwise vs. direct complete excavation, and direct pulp capping vs. partial pulpotomy. Eur J Oral Sci 118(3): 290-297.
- Matsuo T, Nakanshi T, Shimizu H, Ebisu S (1996) A clinical study of direct pulp capping applied to carious exposed pulps. J Endod 22(10): 551-556.
- Silva AF, Tarquinii SBC, Demarco FF, Piva E, Rivero ERC, et al. (2006) The influence of haemostatic agents on healing of healthy human dental pulp tissue capped with calcium hydroxide. Int Endod J 39(4): 309-316.
- Auschill TM, Arweiler NB, Hellwig E, Zamani-Alaei A, Sculean A, et al. (2003) Success rate of direct pulp capping with calcium hydroxide. Swcheiz Monatsschr Zahnmed 113(9): 946-952.
- Bogen G, Chandler N, Ingle JI, Bakland LK, Baumgartner JC (2008) Vital pulp therapy, Ingle's endodontics, (6th edn), Hamilton, BC Decker, Ontario, pp. 1310-1329.
- Elias RV, Dermaco FF, Tarquinio SB, Piva E (2007) Pulp response to the application of a self etching adhesive in human pulps after controlling bleeding with sodium hypochlorite. Quintessence Int 38(2): 67-77.
- Accorinti Mde L, Loguercio AD, Reis A, Muench A, de Araujo VC, et al. (2005) Responses of human pulp capped with a bonding agent after bleeding control with haemostatic agent. Oper Dent 30(2): 147-155.
- Dammaschke T, Stratmann U, Fischer RJ, Sagheri D, Schafer E, et al. (2010) A histologic investigation of direct pulp capping in rodents with dentine adhesives and calcium hydroxide. Quintessence Int 41(4): 6271.
- Gronthos S, Brahim J, Li W (2002) Stem cells properties of human dental pulp stem cells. J Dent Res 81(8): 531-535.
- Baume LJ, Holz J (1981) Long term clinical assessment of direct pulp capping. Int Dent J 31(4): 251-260.
- Hørsted P, S0ndergaard B, Thylstrup A, El Attar K, Fejerskov O, et al. (1985) A retrospective study of direct pulp capping with calcium hydroxide compounds. Endod Dent Traumatol 1(1): 29-34.
- Willershausen B, Willershausen L, Ross A, Velikonja S, Kasaj A, et al. (2011) A retrospective study on direct pulp capping with calcium hydroxide. Quintessence Int 42(2): 165-171.
- Dammaschke T, Leidinger J, Schäfer E (2010) Long term evaluation of direct pulp capping, treatment outcome over an average period of 6.1 years. Clin Oral Investig 14(5): 559-567.
- Cox CF, Sübaby RK, Ostro E, Suzuki S, Suzuki SH (1996) Tunnel defects in dentinal bridges, their formation following direct pulp capping. Oper Dent 21(1): 4-11.
- Bogen J, Kim JS, Bakland LK (2008) Direct pulp capping with mineral trioxide aggregate, an observational study. J Am Dent Ass 139(3): 305315.
- Farsi N, Almoudi N, Balto K, Al Mushayt A (2006) Clinical assessment of mineral trioxide aggregate as direct pulp capping in young permanent teeth. J Clin Pediatr Dent 31(2): 72-76.
- Aeinehchi M, Eslami B, Ghanbariha M, Saffar AS (2003) Mineral trioxide aggregate and calcium hydroxide as pulp capping agents in human teeth, a preliminary report. Int Endod J 36(3): 225-231.
- Min KS, Park HJ, Lee SK (2008) Effect of mineral trioxide aggregate on dentine bridge formation and expression of dentine sialoprotein and heme oxygenase-1 in human dentinal pulp. J Endod 34(6): 666-670.
- Nair PN, Duncan HF, Pitt Ford TR, Luder HU (2008) Histological, ultrastructure and quantitative investigations of healthy human pulp to experimental capping with mineral trioxide aggregate, a randomized clinical trial. Int Endod J 41(2): 128-150.
- Mente J, Gelentneky B, Ohle M (2010) Mineral trioxide aggregate or calcium hydroxide direct pulp capping, an analysis of the clinical treatment outcome. J Endod 36(5): 806-813.
- Olsburgh S, krejci I (2003) Pulp response to traumatic crown fractures. Endod Topic 5(1): 25-40.
- Cvek M (1978) A clinical report on partial pulpotomy and capping with calcium hydroxide in permanent incisors with complicated crown fracture. J Endod 4(8): 232-237.
- Chueh LH, Chiang CP (2010) Histology of irreversible pulpitis premolars treated with mineral trioxide aggregate pulpotomy. Oper Dent 35(3): 370-374.
- Cvek M, Andreasen JO, Andreasen FM, Andreasen L (2007) Endodontic management and use of calcium hydroxide in traumatized permanent teeth. Textbook and color Atlas of traumatic injuries to teeth. (4th edn), Ames, Lowa. Blackwell Munksgaard, USA, pp. 598-654.
- Paranjpe A, Zhang H, Johnson JD (2010) Effect of mineral trioxide aggregate on human dental pulp cells after capping procedure. J Endod 36(6): 1042-1047.
- Mejare I, Cvek M (1993) Partial pulpotomy in young permanent teeth with deep carious lesions. Endod Dent Traumatol 9(6): 238-242.
- Svizero Nda R, Bresciani E, Francischone CE, Franco EB, Pereira JC, et al. (2003) Partial pulpotomy and tooth reconstruction of a crown fractured permanent incisor, a case report. Quintessence Int 34(10): 740-747.
- Qudeimat MA, Barrieshi-Nusair KM, Owais AI (2007) Calcium hydroxide vs mineral trioxide aggregates for partial pulpotomy of permanent molars of deep caries. Eur Arch Pediatr Dent 8(2): 99-104.
- Trope M, Blanco L, Chivian N, Sigurdsson A, Cohen S, et al. (2006) The role endodontics after dental traumatic injuries. Pathway of the pulp, (9th edn), Louis, Mosby, USA.
- Koh ET, Fort TR, Kariyawansam SP, Chen NN, Torabinejad M, et al. (2001) prophylactic treatment of dens evaginatus using mineral trioxide aggregate. J Endod 27(8): 540-542.
- Chacko V, Kurikosa S (2006) Human pulpal reponse to mineral trioxide aggregate, a histological study. J Clin Pediatr Dent 30(3): 203-209.
- El-Meligy OA, Avery DR (2006) Comparison of mineral trioxide aggregate and calcium hydroxide as pulpotomy agents in young permanent teeth (apexogenesis). Pediatr Dent 28(5): 399-404.
- Eghbal MJ, Asgary S, Baglue RA, Parirokh M, Ghoddusi J, et al. (2009) MTA pulpotomy of human permanent molars with irreversible pulpitis. Aust Endod 35(1): 4-8.
- Witherspoon DE, Small JC, Harris GZ (2006) Mineral trioxide pulpotomies, a case series outcome assessment. J Am Dent Ass 137(5): 610-618.
- Sangwan P, Sangwan A, Duhan J, Rohilla A (2013) Tertiary dentinogenesis with calcium hydroxide, a review of proposed mechanisms. Int Endod J 46(1): 3-19.
- Olsson H, Petersson K, Rohlin M (2006) Formation of hard tissue barrier after pulp capping in humans, systematic review. Int Endod J 39(6): 429442.
- Kontham UR, Tiku AM, Damle SG, Kalaskar RR (2005) Apexogenesis of a symptomatic mandibular first permanent molar with calcium hydroxide pulpotomy. Quintessence Int 36(8): 653-657.
- Hørsted-Bindslev P, Løvschall H (2002) Treatment outcome of vital pulp therapy. Endod Topic 2: 24-34.
- Seo MS, Hwang KG, Lee J, Kim H, Baek SH, et al. (2013) The effect of mineral trioxide aggregate on odontogenic differentiation in dental pulp stem cells. J Endod 39(2): 242-248.
- Sarkar NK, Caicedo R, Ritwik P, Moiseyeva R, Kawashima I, et al. (2005) Physiochemical basis of the biologic properties of mineral trioxide aggregate. J Endod 31(2): 97-100.
- Bozeman TB, Lemon RR, Eleazer PD (2006) Elemental analysis of crystal precipitate from gray and white MTA. J Endod 32(5): 425-428.
- Mosoud P, Torabinejad M (2010) Mineral trioxide aggregate, a comprehensive literature review. Part III: Clinical applications, drawbacks and mechanism of action. J Endod 36(3): 400-413.
- Belobrov I, Parashos P (2011) Treatment of tooth discoloration after the use of white mineral trioxide aggregate. J Endod 37(7): 1017-1020.
- De-Deus G, de Souze MC, Sergio Fidel RA, Fidel SR, de Compos RC, et al. (2009) Negligible expression of arsenic in some commercially available brands of Portland cement and mineral trioxide aggregate. J Endod 35(6): 887-890.
- Sheehy EC, Roberts GJ (1997) Use of calcium hydroxide for apical barrier formation and healing in non-vital immature permanent teeth, a review. Br Dent J 183(7): 241-246.
- Chawla HS (1986) Apical closure in non-vital permanent tooth using one calcium hydroxide dressing. J Dent Child 53(1): 44-47.
- Cvek M, Sundstorm B (1974) Treatment of non-vital permanent incisors with calcium hydroxide. V. Histological appearance of roentgenographically demonstrable apical closure of immature roots: Odontotol Revy 25(4): 379-391.
- Barrieshi-Nusair KM, Qudeimat MA (2006) A prospective clinical study of mineral trioxide aggregate for partial pulpotomy in cariously exposed permanent teeth. J Endod 32(8): 731-735.
- Chosack A, Sela J, Cleaton-Jones P (1997) A histological and quantitative histomorphometric study of apexification of non-vital permanent incisors of vervet monkey after repeated root filling with calcium hydroxide paste. Endod Dent Traumatol 13(5): 211-217.
- Yassen GH, Chin J, Mohammedsharif AG, Alsoufy SS, Othman SS, et al. (2012) The effect of frequency of calcium hydroxide dressing changing and varios pre- and inter-operative factors on endodontic treatment of traumatized immature permanent incisors. Dent Traumatol 28(4): 296-301.
- Abbot P (1998) Apexification with calcium hydroxide-when should the dressing be changed? The case for regular dressing changes. Aust Endod J 24(1): 27-32.
- Dominguez Reyes A, Munoz Munoz L, Aznar Martin T (2005) Study of calcium hydroxide apexification in 26 young permanent incisors. Dent Traumatol 21(3): 141-145.
- Fernandez-Yanez, Sanchez A, Leco-Berrocal MI, Martinez-Gonzalez JM (2008) Metaanalysis of filler materials in periapical surgery. Med Oral Pathol Oral Cir Bucal 13(3): E180-E185.
- Chala S, Abouqal R, Rida S (2011) Apexification of immature teeth with calcium hydroxide or mineral trioxide aggregate, systematic review and meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 112(4): e36-e42.
- Bakland LK, Andreasen JO (2012) Will mineral trioxide aggregate replace calcium hydroxide in treating pulpal and periodontal healing complications subsequent to dental trauma, a review. Dent Trauamtol 28(1): 25-32.
- Huang GTJ (2009) Apexification, the beginning of its end. Int Endod J 42(10): 855-866.
- Cvek M (1972) Treatment of non-vital permanent incisors with calcium hydroxide. I Follow up of periapical repair and apical closure of immature roots. Odontotol Revy 23(1): 27-44.
- Feiglin B (1985) Differences in apex formation during apexification with calcium hydroxide paste. Endod Dent Traumatol 1(5): 195-199.
- Sonoyama W, Seo BM, Yamaza T (2007) Human Hertwig's epithelial root sheath cells play crucial roles in cementum formation. J Dent Res 86(7): 594-599.
- Fong CD, Davis MJ (2002) Partial pulpotomy of immature permanent teeth, its present and future. Pediatr Dent 24(1): 29-32.
- Nosrat IV, Nosrat CA (1998) Reparative hard tissue Formation following calcium hydroxide application after partial pulpotomy in cariously exposed pulps of permanent teeth. Int Endod J 31(3): 221226.
- Bakland LK (2000) Management of traumatically injured pulps in immature teeth using MTA. J Calif Dent Assoc 28(11): 855-858.
- Karabucak B, Li D, Lim J, Iqbal M (2005) Vital pulp therapy with mineral trioxide aggregate. Dent Traumatol 21(4): 240-243.
- Bogen G, Kutter S (2009) Mineral trioxide aggregate obturation, a review and case series. J Endod 35(6): 777-790.
- Garcia-Godoy F, Murray PE (2012) Recommendations for using regenerative endodontic procedures in permanent immature traumatized teeth. Dent Traumatol 28(1): 33-41.
- Belobrov L, Weis MV, Parashos P (2008) Conservative treatment of cervical horizontal root fracture and a complicated crown fracture. Aust Dent J 53(3): 260-264.
- Kinirons MJ, Srivasan V, Welburg RR, Finucane D (2001) A study of two centers of variations in the time of apical barrier detection and barrier position in nonvital immature permanent incisors. Int J Pediatr Dent 11: 447-451.
- Finucane D, Kinirons MJ (1999) Nonvital immature permanent incisors: Factors that may influence treatment outcome. Endod Dent Traumatol 15(6): 274-277.
- Sarris S, Tahmassebi JF, Duggal MS, Cross IA (2008) A clinical evaluation of mineral trioxide aggregate for root end closure of non vital immature permanent incisors in children, a pilot study. Dent Traumatol 24(1): 79-85.
- Isidor F, Odman P, Brondum K (1996) Intermittent loading of teeth restored using prefabricated carbon fiber posts. Int J Prosthodont 9(2): 131-136.
- Valois CR, Costa ED (2004) Influence of thickness of mineral trioxide aggregate on scaling ability of root end fillings in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 97(1): 108-124.
- Warner JJ, Al-Salehi SK (2011) Mangement of open apex in a centeral incisor using mineral trioxide aggregate. Dent Update 38(1): 50-54.
- Simon S, Rilliard F, Berdal A, Machtou P (2007) The use of mineral trioxide aggregate in one visit apexification treatment, a prospective study. Int Endod J 40(3): 186-197.
- Bidar M, Disfani R, Gharagozloo S, Khoynezhad S, Rouhani A (2010) Medication With calcium hydroxide improved marginal adaptation of mineral trioxide aggregate apical barrier. J Endod 36(10): 1679-1682.
- Hachmeister DR, Schindler WG, Walker WA (2002) The sealing ability and retention characteristics of mineral trioxide aggregate in amodel of apexification. J Endod 28(5): 386-390.
- Baldassari-Curz LA, Walton RE, Johnson WT (1998) Scanning electron microscopy and histologic analysis of an apexification cap. : a case report. Oral Med Oral Pathol Oral Radio Endod 86(4): 465-468.
- Mente J, Leo M, Panagidis D, Ohle M, Schneider S, et al. (2013) Treatment outcome of mineral trioxide aggregate in open apex teeth. J Endod 39(1): 20-26.
- Pradhan DP, Chawla HS, Gauba K, Goyal A (2006) Comparative evaluation of endodontic management of teeth with unformed apices with mineral trioxide aggregate and calcium hydroxide. J Dent Child 73(2): 79-85.
- El-Meligy OA, Avery DR (2006) Comparison of apexification with mineral trioxide aggregate and calcium hydroxide. Pediatr Dent 28(3): 248-253.
- Grzesilk WJ, Narayanan AS (2002) Cementum and periodontal wound healing and regeneration. Critical Rev Oral Bio Med 13(6): 474-484.
- Solheim E (1998) Growth factors in bone. Int Orthop 22(6): 410-416.
- Khatavkar RA, Hegde VS (2010) Use of matrix for apexification procedure with mineral trioxide aggregate. J Conserv Dent 13(1): 54-57.
- Sood R, Kumar Hans M, Shetty S (2012) Apical barrier technique with mineral trioxide aggregate using internal matrix. A case report. Compend Contin Educ Dent 33(6): e88-e90.
- Cehreli ZC, Sara S, Uysal S, Turgut MD (2011) MTA apical plugs in the treatment of traumatized immature teeth with large periapical lesions. Dent Traumatol 27(1): 59-62.
- Tezal B, Uysal S, Turgut MD, Cehreli ZC (2010) Inadvertent MTA extrusion in immature traumatized permanent incisor. J Clin Pediatr Dent 35(2): 145-148.
- Yates JA (1988) Barrier formation time in non-vital teeth with open apex. Int Endod J 21(5): 313-319.
- Kleier DJ, Barr ES (1991) A study of endodontically apexified teeth. Endod Dent Traumatol 7(3): 112-117.
- Felippe MCS, Felippe WT, Marques MM, Antoniazzi JH (2013) The effect of the renewal of calcium hydroxide paste on the apexification and periapical healing of teeth with incomplete root formation. Int Endod J 38(7): 436-442.
- Twati WA, Wood DJ, Liskiewicz TW, Willmott NS, Duggal MS, et al.(2009) An evaluation of the effect of non-setting calcium hydroxide on human dentine, a pilot study. Eur Arch Pediatr Dent 10(2): 104-109.
- Sahebi S, Moazami F, Abbott P (2010) The effects of short-term calcium hydroxide application on the strength of dentine. Dent Traumatol 26(1): 43-46.
- Hatibovic-Koffman S, Raimundo L, Zheng L, Chong L, Friedman M, et al. (2008) Fracture resistance and histological findings of immature teeth with mineral trioxide aggregate. Dent Traumatol 24(3): 272-276.
- Tuna EB, Dinfol ME, Genfay K, Aktoren O (2011) Fracture resistance of immature teeth filled with Bio Aggregate, mineral trioxide aggregate and calcium hydroxide. Dent Traumatol 27(3): 174-178.
- Yassen GH, Platt JA (2013) The effect of non-setting calcium hydroxide on root fracture and mechanical properties of radicular dentine: a systematic review. Int Endod J 46(2): 112-118.
- Sarkar NK, Caicedo R, Ritwik P, Moiseyeva R, Kawashima I, et al. (2005) Physicochemical basis of the biologic properties of mineral trioxide aggregate. J Endod 31(2): 97-100.
- Al Ansary MA, Day PF, Duggal MS, Brunton PA (2009) Interventions for treating traumatized necrotic immature permanent anterior teeth: inducing a calcific barrier & root strengthening. Dent traumatol 25(4): 367-379.
- Lertchirakarn V, Poonkaew A, Messer H (2011) Fracture resistance of roots filled with gutta-percha or RealSeal®. Int Endod J 44(11): 10051010.
- Weiger R, Heuchert T, Hahn R, Lost C (1995) Adhesion of glass ionomer cement to human radicular dentine. Endod Dent Traumatol 11(5): 214-219.
- Sengun A, Cobankara FK, Orucoglu H (2008) Effect of a new restoration technique on fracture resistance of endodontically treated teeth. Dent traumatol 24(2): 214-219.
- Lawley GR, Schindler WG, Walker WA, Kolodrubetz D (2004) Evaluation of ultrasonically placed MTA and fracture resistance with intracanal composite resin in a model of apexification. J Endod 30(3): 167-172.
- Camp HJ, Fuks AB, Cohen S, Burns RC (2006) Pediatric endodontics: Endodontic treatment for the primary and young permanent dentition. Pathway of the pulp, (9th edn), St Louis, Mosby, USA, p. 874.
- Katebzadeh N, Dalton BC, Trope M (1998) Strengthening immature teeth during and after apexification. J Endod 24(4): 256-259.
- Goldberg F, Kaplan A, Roitman M, Manfre S, Picca M, et al. (2002) Reinforcing effect of a resin modified glass ionomer in the restoration of immature roots in vitro. Dent Traumatol 18(2): 70-72.
- Duret B, Duret F, Reynaud M (1996) Long-life physical property preservation and postendodontic rehabilitation with the composipost. Compend Contin Educ Dent Suppl (20): S50-S56.
- Norsat A, Nekfoofar MH, Bolhari B, Dummer PM (2012) Unintended extrusion of mineral trioxide aggregate, a report of three cases. Int Endod J 45(12): 1165-1176.
- Bose R, Nummikoski P, Hargreaves K (2009) A retrospective evaluation of radiographic outcomes in immature teeth with necrotic root canal systems treated with regenerative endodontic procedures. J Endod 35(10): 1343-1349.
- Chueh LH, Ho YC, Kuo TC, Lai WH, Chen YH, et al. (2009) Regenerative endodontic treatment for necrotic immature permanent teeth. J Endod 35(2): 160-164.
- Thibodeau B (2009) Case report, pulp revascularization of necrotic, infected, immature, permanent tooth. Pediatr Dent 31(2): 145-148.
- Ding RY, Cheung GS, Chen J, Yin XZ, Wang QQ, et al. (2009) Pulp revascularization of immature teeth with apical periodontitis, a clinical study. J Endod 35(5): 745-749.
- Jung IY, Lee SJ, Hargreaves KM (2008) Biologically based treatment of immature permanent teeth with pulpal necrosis, a case series. J Endod 34(7): 876-887.
- Banchs F, Trope M (2004) Revascularization of immature permanent teeth with apical periodontitis, new treatment protocol. J Endod 30(4): 196-200.
- Sato I, Ando-Kurihara N, Kota K, Iwaku M, Hoshino E, et al. (1996) Sterilization of infected root-canal dentine by topical application of a mixture of ciprofloxacin, metronidazole and minocycline in situ. Int Endod J 29(2): 118-124.
- Galler KM, D'Souza RN, Federlin M, Cavender AC, Hartgerink JD, et al. (2011) Dentin conditioning codetermines cell fate in regenerative endodontics. J Endod 37(11): 1536-1541.
- Trevino EG, Patwardhan AN, Henry MA, Perry G, Dybdal-Hargreaves N, et al. (2011) Diogenes Effect of irrigants on the survival of human stem cells of the apical papilla in a platelet-rich plasma scaffold in human root tips. J Endod 37(8): 1109-1115.
- Kim JH, Kim Y, Shin SJ (2010) Tooth discoloration of immature permanent incisor associated with triple antibiotic therapy: a case report. J Endod 36(6): 1086-1091.
- Al-Omiri MK, Mahmoud AA, Rayyan MR, Abu-Hammad O (2010) Fracture resistance of teeth restored with post-retained restorations: an overview. J Endod 36(9): 1439-1449.
- Trope M (2010) Treatment of the immature tooth with a non-vital pulp and apical periodontitis. Dent Clin North Am 54(2): 313-324.
- Reynolds K, Johnson JD, Cohenca N (2009) Pulp revascularization of necrotic bilateral bicuspids using a modified novel technique to eliminate potential coronal discolouration: a case report. Int Endod J 42(1): 84-92.
- Cotti E, Mereu M, Lusso D (2008) Regenerative treatment of an immature, traumatized tooth with apical periodontitis: report of a case. J Endod 34(5): 611-616.
- Morfis AS, Siskos G (1991) Apexification with the use of calcium hydroxide, a clinical study. J Clin Pediatr Dent 16(1): 13-19.
- Cehreli ZC, Isbitiren B, Sara S, Erbas G (2011) Regenerative endodontic treatment (revascularization) of immature necrotic molars medicated with calcium hydroxide: a case series. J Endod 37(9): 1327-1330.
- Huang GTJ (2008) A paradigm shift in endodontic management of immature teeth, conservation of stem cells for regeneration. J Dent 36(6): 379-386.
- Chueh LH, Huang GT (2006) Immature teeth with periradicular periodontitis or abscess undergoing apexogenesis, a paradigm shift. J Endod 32(12): 1205-1213.
- Shin SY, Albert JS, Mortman RE (2009) One step pulp revascularization treatment of an immature permanent tooth with chronic apical abscess, a case report. Int Endod 42(12): 1118-1126.
- Sonoyama W, Liu Y, Yamaza T (2008) Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod 34(2): 166-171.
- Wang X, Thibodeau B, Trope M (2010) Histological characterization of regenerated tissues in canal space after the revitalization/ revascularization procedure of immature dog teeth with apical periodontitis. J Endod 36(1): 56-63.
- Cvek M (1992) Prognosis of luxated non-vital maxillary incisors treated with calcium hydroxide and filled with gutta-percha, a retrospective study. Endod Dent Traumatol 8(2): 45-55.
- Carlson ME, Conboy IM (2007) Loss of stem cell regenerative capacity within aged niches. Aging Cell 6(3): 371-382.
- Huang GT (2009) Pulp and dentin tissue engineering and regeneration, current progress. Regenerative Medicine 4(5): 697-707.
- Nakashima M, Iohara K, Zheng L (2006) Gene therapy for dentin regeneration with bone morphogenetic proteins. Current Gene Therapy 6(5): 551-560.
- Yamauci N, Yamauchi S, Nagaoka H (2011) Tissue engineering strategies for immature teeth with apical periodontitis. J Endod 37(3): 390-397.
- Torabinejad M, Turman M (2011) Revitalization of tooth with necrotic pulp and open apex by using platelet-rich plasma, a case report. J Endod 37(2): 265-268.
- Nosrat A, Seifi A, Asgary S (2011) Regenerative endodontic treatment (revascularization) for necrotic immature permanent molars, a review and report of two cases with a new biomaterial. J Endod 37(4): 562567.
- Kim DS, Park HJ, Yeom JH, Seo JS, Ryu GJ, et al. (2012) Long-term follow- ups of revascularized immature necrotic teeth, three case reports. Int J Oral Sci 4(2): 109-113.
- Yassen GH, Chu TM, Eckert G, Platt JA (2013) Effect of medicaments used in endodontic regeneration technique on the chemical structure of human immature radicular dentin: an in vitro stud. J Endod 39(2): 269-273.
- Iwaya SI, Ikawa M, Kubota M (2001) Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dent Traumatol 17(4): 185-187.
- Thibodeau B, Trope M (2007) Pulp revascularization of a necrotic infected immature permanent tooth, case report and review of the literature. Pediatr Dent 29(1): 47-50.
- Vojinovic O (1981) The endodontic method of treatment of apical periodontitis in immature and young permanent teeth. J Int Ass Dent Child 12: 65-72.
- da Silva L, Nelson-Filho P, da Silva R (2010) Revascularization and periapical repair after endodontic treatment using apical negative pressure irrigation versus conventional irrigation plus triantibiotic intracanal dressing in dogs' teeth with apical periodontitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 109(5): 779-787.
- Chen MY, Chen KL, Chen CA, Tayebaty F, Rosenberg PA, et al. (2012) Responses of immature permanent teeth with infected necrotic pulp tissue and apical periodontitis/abscess to revascularization procedure. Int Endod J 45(3): 294-305.
- Lovelace TW, Henry MA, Hargreaves KM, Diogenes A (2011) Evaluation of the delivery of mesenchymal stem cells into the root canal space of necrotic immature teeth after clinical regenerative endodontic procedure. J Endod 37(2): 133-138.
- Mackie IC, Bentley EM, Worthington HV (1988) The closure of open apices in non-vital immature incisor teeth. Br Dent J 165(5): 167-173.
- Pace R, Giuliani V, Pini Prato L, Baccetti T, Pagavino G, et al. (2007) Apical plug technique using mineral trioxide aggregate. Int Endod J 40(6): 478-484.
- Holden DT, Schwartz SA, Kirkpatrick TC, Schindler WG (2008) Clinical outcome of artificial root end barriers with mineral trioxide aggregate in teeth with immature apices. J Endod 34(7): 812-817.
- Witherspoon DE, Small JC, Regan JD, Nunn M (2008) Retrospective analysis of open apex teeth obdurate with mineral trioxide aggregate. J Endod 34(10): 1171-1176.
- Moore A, Howley MF, O'Connell AC (2011) Treatment of open apex teeth using two types of white mineral trioxide aggregate after initial dressing with calcium hydroxide in children. Dent Traumatol 27(3): 166-173.
- Tahan E, Celik D, Er K, Tajdemir T (2010) Effect of unintentionally extrusion of mineral trioxide aggregate in treatment of tooth with periapical lesion: a case report. J Endod 36(4): 760-763.
- Asmussen E, Peutzfeldt A, Heitmann T (1999) Stiffness, elastic limit and strength of newer types of endodontic posts. J Dent 27(4): 275278.
- Petrino JA, Boda KK, Shambarger S (2010) Challenges in regenerative endodontics, a case series. J Endod 36(3): 536-541.
- Hargreaves K, Geisler T, Henry M, Wang Y (2008) Regeneration potential of the young permanent tooth. what does the future hold? J Endod 34(7 Suppl): S51-S56.
- Hibodeau B, Teixeira F, Yamauchi M, Caplan D, Trope M, et al. (2007) Pulp revascularization of immature dog teeth with apical periodontitis. J Endod 33(6): 680-689.
- Lenzi R, Trope M (2012) Revitalization procedures in two traumatized incisors with different biological outcomes. J Endod 38(3): 411-414.
- Nosrat A, Homayounfar N, Oloomi K (2012) Drawbacks and unfavorable outcomes of regenerative endodontic treatments of necrotic immature teeth, a literature review and report of a case. J Endod 38(10): 14281434.
© 2017 Kareem A Mohamed Kamel, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






