- Submissions

Full Text
Modern Concepts & Developments in Agronomy
Quantitative Modeling and Biological Disruption of Root-Knot Nematodes Through Pasteuria penetrans Endospore Attachment: A Synthesis of Multiple Studies
Ioannis Vagelas*
Department of Agriculture, Laboratory of Plant Pathology, Crop Production and Rural Environment, School of Agricultural Sciences, University of Thessaly, Greece
*Corresponding author:Ioannis Vagelas, Department of Agriculture, Laboratory of Plant Pathology, Crop Production and Rural Environment, School of Agricultural Sciences, University of Thessaly, Greece
Submission: September 03, 2025;Published: September 12, 2025

ISSN 2637-7659Volume15 Issue 3
Abstract
Pasteuria penetrans is an obligatory bacterial parasite of root-knot nematodes (Meloidogyne spp.) and represents a promising biological control agent in sustainable agriculture. A series of studies conducted by Vagelas and co-authors has systematically investigated the statistical modeling, behavioral dynamics and biological efficacy of P. penetrans endospore attachment to second-stage juveniles (J2s). This body of research integrates over dispersed count data analysis, zero-inflated modeling, Markov chain dynamics and in planta validation to elucidate how spore adhesion impairs nematode mobility and infectivity. This review synthesizes the key findings and methodological innovations of Vagelas et al.’s work, offering a predictive framework for the strategic deployment of P. penetrans in sustainable nematode management.
Keywords:Pasteuria penetrans; Obligate endo parasitic bacterium; Spore-forming unculturable microorganism; Biological control; Over dispersed count data; Zero-inflated models; Markov chain modeling; Spore attachment thresholds; Sustainable nematode management
Background
Root-knot nematodes (Meloidogyne spp.) are among the most economically damaging plant-parasitic nematodes, affecting a wide range of crops worldwide. Their ability to invade root tissues, induce gall formation, and disrupt nutrient uptake makes them a persistent threat to agricultural productivity. Although chemical nematicides have historically been used to manage infestations, their environmental impact and regulatory limitations have prompted the search for sustainable alternatives. Pasteuria penetrans (Pp), an obligate bacterial parasite of Meloidogyne spp., has emerged as a promising biological control agent due to its ability to attach to the nematode cuticle, inhibit movement, prevent root invasion, and ultimately sterilize adult females. However, the effectiveness of P. penetrans is closely tied to the number of endospores adhering to each juvenile nematode (J2) and the biological variability in attachment rates has posed challenges for consistent field application [1-7]. To address these challenges, Dr. Ioannis Vagelas and co-authors have developed a comprehensive research framework that integrates statistical modeling, behavioral analysis and in planta validation. The main objectives of Vagelas and co-authors’ work include quantifying spore attachment dynamics using negative binomial and zero-inflated models, simulating nematode movement through Markov chain analysis, linking in vitro observations to actual parasitism outcomes in tomato plants and developing predictive tools for biological control deployment [8-10]. Their studies also incorporate image-based tracking of nematode motility, explore the influence of environmental factors such as soil texture and exposure time and establish biologically meaningful thresholds-such as the ≥7 spores per J2 benchmark-for effective suppression. This synthesis presents the core findings and innovations across six major studies, demonstrating how mathematical ecology and computational biology can enhance the precision and reliability of microbial biocontrol strategies. Moreover, by translating biological variability into predictive thresholds, this research bridges laboratory insights with field-level implementation.
Materials and Methods
This review synthesizes the research conducted by Dr. Vagelas and co-authors, with particular emphasis on the biological control efficacy of P. penetrans against root-knot nematodes (Meloidogyne spp.).
Nematode culture and spore preparation
Cultures of Meloidogyne javanica were maintained on tomato plants (cv. Tiny Tim) in greenhouse conditions. Eggs were extracted using a 0.5% sodium hypochlorite solution and passed through nested sieves (200-mesh over 500-mesh) to isolate viable eggs. Second-stage juveniles (J2s) were hatched under standard laboratory conditions. Commercial spore suspensions of Pasteuria penetrans (Nematech Co. Ltd., Japan) were prepared in tap water or tap water mixed with loam soil, depending on the bioassay [8].
In vitro attachment bioassays
Freshly hatched J2s were exposed to 5000 spores per Petri dish (2.5cm diameter) and incubated at 28 °C. Attachment counts were recorded at multiple time points (1, 3, 6, 9, 12, 24, 48 and 96 hours). Observations were conducted under an inverted microscope at ×200 magnification. For each point, 36-100 nematodes were examined across different treatments [8].
Image-based tracking and motility analysis
J2 movement was recorded using an inverted microscope (MICROTEC 200) equipped with a digital camera (Aiptek 3M). Videos were broken down into 400 frames per 40-second sequence with SC Video Decompiler. Image J software tracked the centroid of each nematode body and measured displacement, body posture, and movement paths. J2s were sorted into three groups: Unencumbered, Low-Pp (5-8 spores) and High-Pp (20-30 spores) [11].
Markov chain modeling
Directional turns of the nematode cephalic region were classified as LL, LR, RL, or RR transitions (e.g., left/right head turns). Transition matrices (P for unencumbered J2s, Q for encumbered J2s) were constructed based on 20 sequential frames per nematode. Steady-state probabilities (P256 and Q256) were calculated using a TI-83 plus graphing calculator and analyzed in excel [12,13].
In planta experiments
Tomato plants (4 weeks old) were inoculated with 300 ± 32 J2s per plant. Treatments included: Control (no spores), Low- Pp, and High-Pp. Plants were grown in a glasshouse at 26 °C and evaluated after 25 days. These thresholds, Low-Pp and High-Pp, were selected based on prior observations of behavioral disruption and parasitism failure. Parameters recorded included the number of females, root galls and egg masses per plant. Each treatment was replicated 16 times [14].
Statistical analysis
Count data were analyzed using STATA 9.1 and Best Fit 3.0. Models included Poisson, Negative Binomial (NB), Zero-Inflated Poisson (ZIP) and Zero-Inflated Negative Binomial (ZINB) [9]. Goodness-of-fit was assessed using chi-square tests, likelihood ratios, and Vuong tests [9]. Nonlinear regression and odds ratio analysis were also applied to evaluate parasitism thresholds.
Result
In Vagelas’s research, several key findings emerged that advanced our understanding of how P. penetrans disrupt the movement and infectivity of root-knot nematodes (Meloidogyne spp.):
Statistical modeling of spore attachment
Quantitative analysis revealed that spore attachment data were highly over dispersed, with variance exceeding the mean. Overdispersion indicates that nematode responses vary widely, necessitating flexible models that account for biological heterogeneity. To address this, Vagelas employed the Negative Binomial (NB) distribution, which outperformed the Poisson model in goodness-of-fit metrics. In datasets with an excess of zeros-representing J2s with no spore attachment-Zero-Inflated Negative Binomial (ZINB) models demonstrated superior predictive accuracy. These results were validated using Vuong tests and likelihood ratio comparisons, confirming the robustness of the statistical framework for modeling attachment dynamics [9]. These models enabled probabilistic forecasting of nematode infectivity based on spore load thresholds. Notably, attachment of seven or more spores (≥7) per juvenile (J2) was consistently associated with a significant reduction in root invasion (Table 1), establishing a biologically meaningful benchmark for effective parasitism. This threshold serves as a practical guide for field-level implementation of P. penetrans as a microbial biocontrol agent.
Table 1:Thresholds of Pasteuria penetrans spore attachment affecting nematode movement and plant infection.

Dynamic modeling of nematode movement
In a pioneering approach, Vagelas employed Markov chain models to simulate the rotational behavior of the nematode cephalic region. By categorizing directional turns (LL, LR, RL, RR) and constructing transition matrices, he demonstrated that sporeencumbered second-stage juveniles (J2s) exhibited disorganized movement patterns. These were characterized by an increased probability of repetitive turning and diminished directional persistence. This study represents the first known application of Markov chains to quantify the disruption of nematode locomotion caused by microbial attachment, specifically by P. penetrans endospores [13]. Digital image analysis using centroid tracking (via Image J) provided quantitative evidence of reduced motility in nematode juveniles (J2s) encumbered with P. penetrans spores [8,9]. J2s with high spore loads (20-30 spores) exhibited minimal displacement and erratic trajectories, in contrast to unencumbered nematodes, which maintained linear and purposeful movement. These findings support the hypothesis that P. penetrans exert a nematostatic effect, impairing host locomotion through both mechanical interference and behavioral disruption.
In-planta validation of biological control
Greenhouse trials confirmed the in vitro predictions, demonstrating that tomato plants inoculated with J2s carrying high P. penetrans spore loads developed significantly fewer root galls, females and egg masses compared to untreated controls. The biological impact was dose-dependent: J2s with 5-8 spores showed partial suppression, whereas those with 20-30 spores were nearly incapable of establishing infection [13]. These findings confirm that statistical thresholds derived from laboratory models-such as the ≥7 spores per J2 benchmark-are biologically meaningful and predictive under greenhouse conditions. This correlation between modeled spore attachment and observed parasitism underscores the ecological validity of the statistical and dynamic frameworks. Moreover, it establishes a practical threshold for the field application of P. penetrans as a reliable biocontrol agent.
Comparative summary of Vagelas’s research contributions
The following table (Table 2) presents a comparative summary of five key studies authored by Dr. Vagelas, each contributing uniquely to the understanding of P. penetrans as a biological control agent against root-knot nematodes (Meloidogyne spp.). These studies span over a decade and collectively demonstrate progressive advancements in statistical modeling, behavioral analysis, and biological validation. Each study introduces a unique methodological innovation, ranging from the modeling of over dispersed count data using negative binomial distributions to the simulation of nematode movement via Markov chain analysis and the in-planta validation of predictive thresholds. The implementation of zero-inflated models effectively addresses the challenge of excessive zeros in biological datasets. Additionally, image-based tracking and centroid analysis provide robust quantitative insights into nematode motility under spore encumbrance [9].
Table 2:Comparative summary of Vagelas’s research contributions on Pasteuria penetrans as a biocontrol agent.
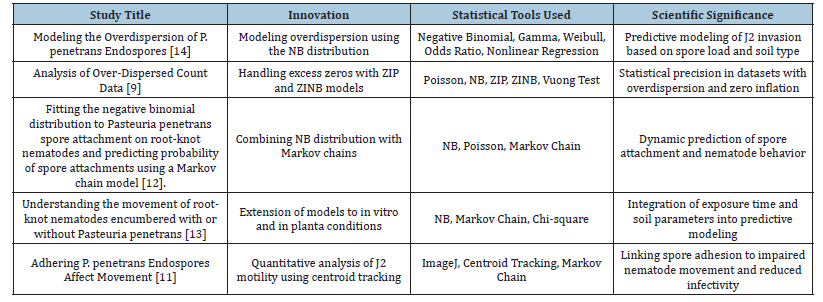
Together, these contributions form a cohesive framework [13] that integrates statistical rigor with biological relevance. Collectively, they offer predictive tools for estimating spore attachment thresholds, assessing nematode infectivity, and optimizing the deployment of P. penetrans in agricultural systems. Table 2 presents a comparative summary of the core innovations, analytical methodologies and scientific significance of each publication, highlighting the progressive evolution of Dr. Vagelas’s research and its impact on microbial biocontrol strategies. Dr. Ioannis Vagelas and co-authors authored all studies listed in Table 2 between 2012 and 2022.
Scientific significance and innovation
The collective body of work by Vagelas et al. represents a
significant methodological advancement in the fields of nematology
and biological control. These studies introduce and refine a suite
of analytical and experimental innovations that have reshaped how
microbial agents like P. penetrans are understood and applied:
a. Advanced statistical modeling using Negative Binomial (NB)
and Zero-Inflated Negative Binomial (ZINB) distributions to
account for biological variability and over dispersed data.
b. Application of Markov chain analysis to quantify and simulate
nematode movement disruption caused by spore attachment.
c. Integration of image-based tracking and centroid analysis for
precise, quantitative assessment of nematode motility.
d. Validation of in vitro predictions through controlled in planta
experiments, confirming the ecological relevance of statistical
thresholds.
e. Development of predictive tools to guide field-level deployment
of microbial biocontrol agents, enhancing reliability and
scalability.
Together, these contributions exemplify how mathematical ecology and computational biology can deepen our understanding of host-pathogen dynamics and support the sustainable implementation of microbial biocontrol strategies in agriculture.
Integrated statistical and biological modeling of p. penetrans spore attachment and its impact on root-knot nematode suppression
Experimental data from multiple studies by Vagelas et al. demonstrate that P. penetrans spore attachment levels exert a direct influence on both the mobility of Meloidogyne juveniles (J2s) and their ability to infect host plants. Motility begins to deteriorate when J2s are encumbered with 5-8 spores, with observable disruptions in directional movement. Root invasion is significantly suppressed at spore loads of ≥7 spores per J2, establishing a biologically meaningful threshold for effective parasitism. At ≥15 spores, nematodes are unable to invade or reproduce, indicating complete biological control under experimental conditions (Table 1).
Conclusion
Dr. Vagelas’s research demonstrates that the attachment of P. penetrans endospores to root-knot nematode juveniles is not only quantifiable but also predictive of biological control efficacy. The integration of statistical distributions, zero-inflated models and Markov chains establishes a robust and interdisciplinary framework for understanding and forecasting nematode behavior and parasitism dynamics. The consistent finding across multiple studies [8,12,14], that ≥7 spores per J2 significantly suppresses root invasion, provides a biologically meaningful and practically applicable threshold for field application. Furthermore, the observed disruption of nematode movement due to spore encumbrance substantiates the nematostatic effect of P. penetrans, reinforcing its role as a potent and sustainable biocontrol agent in agricultural systems.
Future Research Directions
To build upon the foundational contributions of Vagelas et
al., future studies should aim to expand and refine the integrated
framework for microbial biocontrol by addressing the following
key areas:
a. Incorporating soil physicochemical parameters-such as pH,
moisture content, and texture-into predictive models to better
reflect field-level variability and enhance ecological relevance.
b. Investigating host plant genotype interactions, particularly
how genetic variation influences spore attachment efficiency
and nematode suppression across crop species.
c. Advancing real-time image-based tracking technologies for
automated analysis of nematode behavior, enabling highthroughput
and objective quantification of motility patterns.
d. Validating statistical and behavioral models under field
conditions across diverse agroecosystems to assess robustness,
scalability and practical applicability.
e. Exploring the long-term persistence and recycling of Pasteuria
penetrans spores within soil microbiomes, including
their interactions with native microbial communities and
environmental stressors.
f. Integrating Bayesian inference and machine learning
algorithms to improve predictive accuracy, model adaptability
and the development of decision-support tools for precision
biocontrol deployment.
References
- Davies KG, Kerry BR, Flynn CA (1988) Observations on the pathogenicity of Pasteuria penetrans, a parasite of root-knot nematodes. Annals of Applied Biology 112(3): 491-501.
- Channer AG, Gowen S, Tzortzakakis EA (1998) Control of the root-knot nematode Meloidogyne javanica by the parasite Pasteuria penetrans as influenced by the initial nematode population densities. Nematologica 44: 369-379.
- Gowen S, Ahmed R (1990) Pasteuria penetrans for control of pathogenic nematodes. Aspects of applied biology, pp. 25-32.
- Davies KG (2009) Understanding the interaction between an obligate hyper parasitic bacterium, Pasteuria penetrans and its obligate plant-parasitic nematode host, Meloidogyne spp. Advances in Parasitology 68: 211-245.
- Vagelas IK (2015) Novel bacteria species in nematode biocontrol. Biocontrol Agents of Phytonematodes, pp. 310–320.
- Abd-Elgawad MMM, Vagelas IK (2015) Nematophagous bacteria: Field application and commercialization. Biocontrol Agents of Phytonematodes, pp. 276-309.
- Shahid MI, Gowen S, Pembroke B (2021) Effect of spore attachment methods and levels on invasion and parasitism of root-Knot nematode Meloidogyne javanica. Plant Protection 5(2): 75-81.
- Vagelas IK, University of Reading Department of Mathematics and Statistics, University of Reading School of Mathematical and Physical Sciences (2011a) Analysis of the movement of juvenile root-knot nematodes encumbered with spores of Pasteuria penetrans. MPhil dissertation.
- Vagelas I, Pembroke B, Gowen S (2011b) Techniques for image analysis of movement of juveniles of root-knot nematodes encumbered with Pasteuria penetrans Biocontrol Science and Technology 21(2): 239-250.
- Vagelas I (2022) Analysis of over-dispersed count data: Application to obligate parasite Pasteuria Penetrans. WSEAS Transactions on Environment and Development 18: 333-339.
- Vagelas I, Dennett MD, Pembroke B, Gowen S (2012) Adhering Pasteuria penetrans endospores affect movements of root-knot nematode juveniles. Phytopathologia Mediterranea 51(3): 618-624.
- Vagelas I, Dennett MD, Pembroke B, Gowen S (2013a) Fitting the negative binomial distribution to Pasteuria penetrans spore attachment on root-knot nematodes and predicting probability of spore attachments using a Markov chain model. Biocontrol Science and Technology 23(11): 1296-1306.
- Vagelas I, Dennett MD, Pembroke B, Ipsilandis PG, Gowen S (2013b) Understanding the movement of root-knot nematodes encumbered with or without Pasteuria penetrans. Biocontrol Science and Technology 23: 92-100.
- Vagelas I, Leontopoulos S (2022) Modeling the overdispersion of Pasteuria penetrans Parasitologia 3: 206-227.
© 2025 Ioannis Vagelas. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






