- Submissions

Full Text
Modern Concepts & Developments in Agronomy
Bellis Perennis as a Host of Virus, A Worldwide First Record
Rathore A1,2, Diwedi R1, Maurya PK1, Vijayanandraj S1, Chauhan PS3 and Kumar S1*
1Plant Molecular Virology Laboratory, India
2Academy of Scientific and Innovative Research (AcSIR), India
3Microbial Technology Division, India
*Corresponding author:Kumar S, Plant Molecular Virology Laboratory, CSIR-National Botanical Research Institute, Rana Pratap Street, PO 436, Lucknow-226001, Uttar Pradesh, India
Submission: August 22, 2024;Published: October 24, 2024

ISSN 2637-7659Volume14 Issue 4
Abstract
Bellis perennis is used across the globe in garden landscaping and as a pot plant for beautification besides various cosmeceuticals and medicinal properties, and therefore has significant agronomic value. B. perennis plants were observed exhibiting yellow vein net symptom in gardens at Lucknow during a roving survey. The size of foliage and bloom was smaller than the healthy plants growing around, in diseased plants. Ageratum (weed) plants in the same gardens were also observed showing similar yellow vein net symptom. The infection of begomovirus was confirmed in these plants by PCR and full-length DNA-A and betasatellite genomes inferred. The DNA-A revealed 87.6-95.9% nucleotide sequence identity with Ageratum Enation Virus (AEV), whereas betasatellite DNA revealed 85.9-95.0% identity with Ageratum Leaf Curl Betasatellite (ALCB) and clustered with them in phylogeny. No viral disease is known in B. perennis world over before this investigation. To the best of our knowledge, this is the first ever record of AEV and ALCB occurrence in B. perennis that may serve as reservoir for further virus dissemination in other argonomically important plants and need attention
Keywords:
Lawn-daisy; Begomovirus; Yellow vein net; Virus disseminationIntroduction
Bellis perennis L. (family Asteracea) is a perennial herb and widely naturalized in temperate regions of the world including Europe, Northern America, and Central Asia. The plants bloom from early to mid-summer and long flowering season, even produce a few flowers until the middle of mild winter, and therefore used for decoration in landscaping and also as pot plant. B. perennis has astringent properties and has also been used in herbal medicine. Fresh, fully expanded leaves are consumed raw in salads or cooked, petal and flower buds used raw in sandwiches, soups, tea as a vitamin supplement (“Bellis perennis L”. Plants for a Future database). The crude extract or a fraction of B. perennis has properties like cosmeceuticals [1], therapeutic effects on common colds, wound healing, and antitumor, [2,3], as an antioxidant [4,5], anticancerogenic [6], antimicrobial [7], antidepressive and anxiolytic [8], nephroprotective, and insulin mimetic effects [9], as well as an effect on lipid metabolism [10]. B. perennis plant system has also been used as a biotechnological tool for protein localization studies where biolistic transfection of heme-oxygenase tagged with fluorescent reporter was established in epidermal pavement cells containing a moderate number of functional chloroplasts [11]. Literature mining revealed only a handful of records of pathogen attack on B. perennis [12] but no virus disease reported world over. Here we report the hitherto unknown yellow vein net disease in B. perennis, a threat to it and other economically important crops.
Materials and Methods
Young leaf samples of B. perennis exhibiting yellow vein net symptoms along with asymptomatic (healthy) leaf were collected from Lucknow (26.8563° N, 80.9499° E) and subjected for virus detection. Leaf samples from ageratum weed plants exhibiting similar yellow vein net symptoms, growing in the vicinity, were also collected. For all the virus transmission studies, healthy plants of B. perennis of 3-4 leaf stage were used. For molecular detection of virus, total genomic DNA was isolated from 100mg of symptomatic and asymptomatic leaf samples of B. perennis and ageratum using DNeasy Plant Mini Kit (QIAGEN, GmbBH, Germany). The universal primer pairs: PAL1v1978/ PAR1c496 [13] and Beta01/02 [14] were used for begomovirus detection. Briefly, reaction was carried out in 50μl reaction mixture that included 50ng of template DNA, 1x Pfu DNA polymerase buffer, 25pM each of forward and reverse primers, 200μM dNTPs, 25mM MgCl2, and 3 U Pfu DNA polymerase (MBI Fermentas, NY, USA). Amplification was carried out in a Peltier thermal cycler- PTC200 (MJ Research, Waltham, MA, USA) following the conditions described elsewhere [13,14]. PCR products were size separated on a 1% agarose gel and size assessed using a DNA marker (Lambda DNA digested with EcoRI/HindIII; Genei, Bangalore, India).
The circular viral genome was amplified by Rolling Circle Replication (RCR) method using Ø-29 DNA polymerase from genomic DNA using TempliPhi Amplification kit (GE Healthcare, NJ, USA) following the standard procedure [15]. The RCA product was monomerized by digestion with XbaI, XhoI and BamHI (New England BioLabs, USA) as described earlier [16], cloned in pCAMBIA2300 binary vector backbone at respective endonuclease digestion sites and get sequenced (Genei, Bangalore, India). Similarity searches were carried out with the BLASTn search program (http://www. ncbi.nlm.nih.gov/BLAST/). Phylogenetic trees were created using MEGA software v.11 [17] and similarity scores were generated using SDT v1.3 [18].
Whitefly-mediated virus transmission assay was performed to satisfy Koch’s postulates as previously described [16]. Aviruliferous whiteflies (Bemisia tabaci complex) reared on healthy tomato plants were collected, starved for 3 hours, and then allowed to feed for 24 hours on an infected B. perennis. Ten viruliferous whiteflies (each plant) were fed on healthy B. perennis saplings grown in insect-proof cage.
Result and Discussions
About 21% (29 out of 139) B. perennis plants were observed displaying distinct yellow vein net symptoms as compared to the healthy plants growing in gardens (Figure 1a). In diseased plants, the size of leaf and flower was smaller than the healthy ones growing around. Plants were heavily infested by whiteflies. In the same plot, ageratum plants growing as weeds were also observed exhibiting yellow vein net symptoms (Figure 1b). The occurrence of whitefly in the vicinity and yellow vein net symptom on B. perennis and ageratum suggested the infection of begomovirus.
Figure 1:Image showing filed grown naturally infected Bellis perennis plant (circled in white) exhibiting yellow vein net symptom on leaves and healthy plants around (a). Ageratum plant growing near the ornamental field exhibiting yellow vein net symptom (b).

PCR with PALIv722/PALIc1960 and beta01/beta02 primers resulted in amplification of expected size of 1.2kb and 1.3bp DNA bands corresponding to size of begomovirus DNA-A and betasatellite, respectively in all infected B. perennis and ageratum plants. The complete DNA-A and betasatellite genomes were amplified by RCR method which revealed about 2.7 and 1.3kb DNA bands by digestion with XbaI and HindIII restriction endonucleases. These DNAs were independently cloned in pCAMBIA2300 at respective sites, sequenced and submitted (NCBI Genbank accession number PP524973 and PP524974). The DNA-A genome (PP524973) revealed 87.6% to 95.9% nucleotide sequence identity with the previously identified Ageratum Enation Virus (AEV) whereas betasatellite DNA (PP524974) revealed 85.9% to 95.0% sequence identity with Ageratum Leaf Curl Betasatellite (ALCB) in BLASTn and clustered with them during phylogeny.
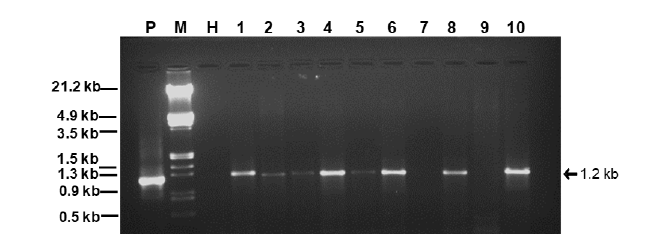
Koch’s postulate was satisfied (to ascertain the AEV and associate ALCB as the cause of yellow vein net disease in naturally infected B. perennis) by whitefly-mediated virus transmission assay. The healthy B. perennis seedlings developed similar symptoms as were in the naturally infected plants 45-days post whitefly transmission. The presence of virus in these plants was confirmed by PCR with PALIv722/PALIc1960 primers showing the amplification of 1.2kb DNA band in 8 out of 10 inoculated plants (Figure 2), suggesting the fulfilment of Koch’s postulates.
Figure 2:1% Agarose gel images showing 1.2kb DNA bands for AEV in ten randomly selected B. perennis plants (lane = 1-10) at 45-days post whitefly transmission. P = positive control (naturally infected B. perennis), H = healthy B. perennis, M = Lambda DNA Marker (EcoRI/HindIII digested).
The exhaustive literature mining revealed only a handful of records of the pathogen attack on B. perennis. Garibaldi et al. [12] reported the occurrence of powdery mildew caused by Golovinomyces cichoracearum on B. perennis in commercial farms at Albenga (northern Italy) where infected plants were covered with white mycelia and conidia and displaying severe yellowing symptoms. The leaf spot disease of B. perennis caused by Alternaria alternata was reported in Shandong Province, China [19] where symptoms on the initially infected leaves manifested as light yellow, round or oval lesions with light or brown borders, which gradually expanded, deepened in color, and became irregular in shape as the disease progressed. However, no such disease symptoms and occurrence of any virus on B. perennis is reported hitherto. The discovery of this new disease is beneficial to the application and protection of B. perennis which is a popular landscape and medicinal plant.
Acknowledgement
We are thankful to the Director, CSIR-NBRI, Lucknow for providing necessary research facilities and fund through OLP- 117. Author A. Rathore is thankful to DST-Inspire-JRF (IF21047) fellowship. Institutional manuscript number is CSIR-NBRI_ MS/2024/09/07.”
References
- de Carvalho VMS, Covre JL, Correia-Silva RD, Lice I, Corrêa MP, et al. (2021) Bellis perennis extract mitigates UVA-induced keratinocyte damage: Photoprotective and immunomodulatory effects. Journal of Photochemistry and Photobiology B: Biology 221: 112247.
- Karakaş FP, Karakaş A, Boran Ç, Türker AU, Yalçin FN, et al. (2012) The evaluation of topical administration of Bellis perennis fraction on circular excision wound healing in wistar albino rats. Pharmaceutical Biology 50(8): 1031-1037.
- Morikawa T, Ninomiya K, Takamori Y, Nishida E, Yasue M, et al. (2015) Oleanane-type triterpene saponins with collagen synthesis-promoting activity from the flowers of Bellis perennis. Phytochemistry 116: 203-212.
- Siatka T, Kašparová M (2010) Seasonal variation in total phenolic and flavonoid contents and DPPH scavenging activity of Bellis perennis flowers. Molecules 15(12): 9450-61.
- Kavalcioğlu N, Açik L, Demirci F, Demirci B, Demir H, et al. (2010) Biological activities of Bellis perennis volatiles and extracts. Natural Product Communications 5(1): 147-50.
- Ninomiya K, Motai C, Nishida E, Kitagawa N, Yoshihara K, et al. (2016) Acylated oleanane-type triterpene saponins from the flowers of Bellis perennis show anti-proliferative activities against human digestive tract carcinoma cell lines. Journal of Natural Medicines 70(3): 435-451.
- Avato P, Vitali C, Mongelli P, Tava A (1997) Antimicrobial activity of polyacetylenes from Bellis perennis and their synthetic derivatives. Planta Medica 63(6): 503-507.
- Marques THC, De Melo CHS, De Carvalho RBF, Costa LM, De Souza AA, et al. (2013) Phytochemical profile and qualification of biological activity of an isolated fraction of Bellis perennis. Biological Resesarch 46(3): 231-238.
- Haselgrübler R, Stadlbauer V, Stübl F, Schwarzinger B, Rudzionyte I, et al. (2018) Insulin mimetic properties of extracts prepared from Bellis perennis. Molecules 23(10): 2605.
- Albien AL, Stark TD (2023) (Bio)active compounds in daisy flower (Bellis perennis). Review Molecules 28(23): 7716.
- Jaedicke K, Rösler J, Gans T, Hughes J (2011) Bellis perennis: a useful tool for protein localization studies. Planta 234(4): 759-68.
- Garibaldi A, Minuto A, Gullino ML (2008) First report of powdery mildew caused by Golovinomyces cichoracearum on English daisy (Bellis perennis) in Italy. Plant Disease 92(3): 484.
- Rojas MR, Gilbertson RL, Russel DR, Maxwell DP (1993) Use of degenerate primers in the polymerase chain reaction to detect whitefly transmitted geminiviruses. Plant Disease 77: 340-347.
- Briddon RW, Bull SE, Mansoor S, Amin I, Markham PG (2002) Universal primers for the PCR-mediated amplification of DNA beta: a molecule associated with some monopartite begomoviruses. Molecular Biotechnology 20(3): 315-318.
- Guenoune-Gelbarta D, Sufrin-Ringwalda T, Capobiancob H, Gabac V, Polstonb JE, et al. (2010) Inoculation of plants with begomoviruses by particle bombardment without cloning: Using rolling circle amplification of total DNA from infected plants and whiteflies. Journal of Virological Methods 168(1-2): 87-93.
- Srivastava A, Raj SK, Kumar S, Snehi SK, Kulshreshtha A, et al. (2013) Molecular identification of Ageratum enation virus, betasatellite and alphasatellite molecules isolated from yellow vein diseased Amaranthus cruentus in India. Virus Genes 47(3): 584-590.
- Tamura K, Stecher G, Kumar S (2021) MEGA11: Molecular Evolutionary Genetics Analysis version 11. Molecular Biology and Evolution 38(7): 3022-3027.
- Muhire BM, Varsani A, Martin DP (2014) SDT: A virus classification tool based on pairwise sequence alignment and identity calculation. PLoS One 9(9): e108277.
- Wang H, Gao J, Wang Y, Wu Q, Fan M, et al. (2022) First report of Alternaria alternata causing leaf spot on Bellis perennis in China. Plant Disease 106(12): 3210.
© 2024 Kumar S. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






