- Submissions

Full Text
Modern Concepts & Developments in Agronomy
Collecting Times and Sterilization Methods Affect Tissue Culture of Rare and Endangered Species from Western Alps
Matteo Caser1, Ivan Pace2 and Paola Maria Chiavazza1*
1Department of Agricultural, Forest and Food Sciences, University of Torino, Largo Paolo Braccini 2, 1009, Grugliasco (TO), Italy
2Ente di Gestione Aree Protette Alpi Marittime, Piazza Regina Elena, 30, I-12010 Valdieri (CN), Italy
*Corresponding author:Paola Maria Chiavazza, Department of Agricultural, Forest and Food Sciences, University of Torino, Largo Paolo Braccini 2, 1009, Grugliasco (TO), Italy
Submission: November 20, 2023;Published: December 06, 2023

ISSN 2637-7659Volume13 Issue 4
Abstract
Climate change and human actions are compromising the conservation status of natural habitats, spontaneous plants, and animal species. To cope with these changes, the European Union has set up the Natura 2000 Network, a network of sites of community interest and special protection areas created for the protection and conservation of habitats, animal and plant species biodiversity. Among the methods used to conserve biodiversity, micropropagation is an in vitro culture method of plant tissues. The aim of this study was to examine the effect of different collecting times and sterilization methods with the potential to enhance in vitro performance for ex situ conservation of six rare and endangered plant species of the Ligurian and Maritime Alps (Clematis alpina (L.) Miller, Dracocephalum ruyschiana L., Gentiana asclepiadea L., Hyssopus officinalis L., Phyteuma cordatum Balb. and Ruscus hypoglossum L.). Results showed that for both C. alpina and D. ruyschiana, as later in the summer were collected explants as higher values in established explants with shoot induction. While the use of plant preservative mixture in tissue culture media was not effective in increasing explant establishment in the studied species. The findings raise concerns regarding the most promosing in vitro protocols for the multiplication of rare and endangered alpine species.
Keywords:Endangered plants; GD medium; In vitro; Micropropagation; Rare plants; Red lists plants; Regeneration
Introduction
The loss of gene pool of any species is an irreversible damage to the biological diversity of the Earth. Therefore, the conservation of wild rare and endangered plant species is a priority task. At the same time, many plant species that are under threat of extintion are considered as sink for pharmaceuticals, nutraceuticals, cosmetic, and perfumery products [1]. Climate change and human actions are compromising the conservation status of natural habitats and spontaneous plant species, particularly from alpine areas. To cope with these changes, the European Union has set up the Natura 2000 Network, a network of Sites of Community Interest (SIC) and Special Protection Areas (SPA) created in the regulatory framework of the “Habitats Directive (92/43/EEC) and the Birds Directive” (79/409/EEC), for the protection and conservation of habitats, animal and plant species biodiversity [2].
Biotechnological methods are becoming increasingly important for the conservation and exploitation of plant species. Among the methods used to conserve biodiversity, micropropagation is a multiplication technique that allows to obtain a clone of the plant or a set of individuals with the same genetic heritage, using in vitro culture methods of plant tissues. The most important part of any experiment involving plant tissue culture is the seasonal source of the plants and the target surface-sterilization method; wastage of plant cultures, labour and time may result from errors performed at this stage [3]. In fact, the season of explant collection can play a pivotal role in the in vitro establishment of initial cultures starting from adult mother plants [4]. Another major concern in plant tissue culture is the occurrence of in vitro contamination due to the natural presence of bacteria or fungi upon the surfaces and natural openings of the target tissue [3]. In order to reduce the occurrence of such contaminations and boost plant survival, the highest priority is placed on the development and pairing of both surface sterilization recipes and efficient aseptic techniques prior to their execution on the target explant [5]. Isothiazolones are a class of industrial biocides that have been used in the form of plant preservative mixture (PPM™) in tissue culture media to control microbial contamination [6].
Therefore, this study aims to examine the effect of different collecting times and sterilization methods with the potential to enhance in vitro performance for ex situ conservation of six rare and endangered plant species of the Ligurian and Maritime Alps (Clematis alpina (L.) Miller, Dracocephalum ruyschiana L., Gentiana asclepiadea L., Hyssopus officinalis L., Phyteuma cordatum Balb. and Ruscus hypoglossum L.) present within Natura 2000 Network.
Materials and Methods
Plant material
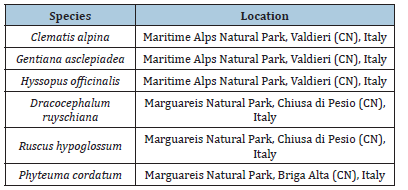
Adult plants of Clematis alpina (L.) Miller, Dracocephalum ruyschiana L., Gentiana asclepiadea L., Hyssopus officinalis L., Phyteuma cordatum Balb. and Ruscus hypoglossum L. were collected in their natural environment (Table 1).
Table 1:Collecting site of the studied species.
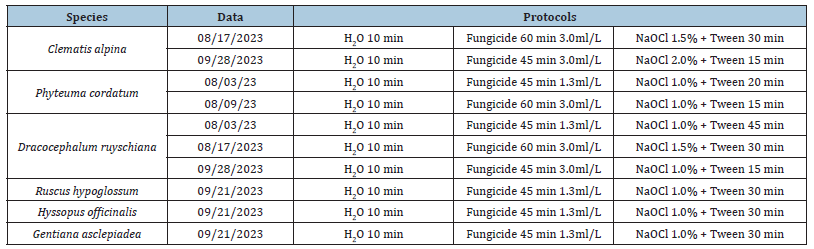
Explants about 8/10cm long were collected from a few adult plants. To ensure adequate genetic variability and maintain the right humidity, the material taken from some individuals has been stored in plastic bags. This material was stored at 4 °C for a few days before being used in the laboratory. Explants of 1-2 centimeters long, each containing a vegetative apex were placed in containers for washing in cold running water (10 min). Subsequently, sterilization operations were carried out for the in vitro culture. To face fungal pollution, a fungicide (Enovit Methyl FL) was applied in association with NaOCl. To identify the right concentrations, various tests were carried out at different concentrations and exposure times. In Table 2 are reported the applied sterilization protocols.
Table 2:Sterilization protocols used for each species in the different trials.
Collecting time and sterilization methods
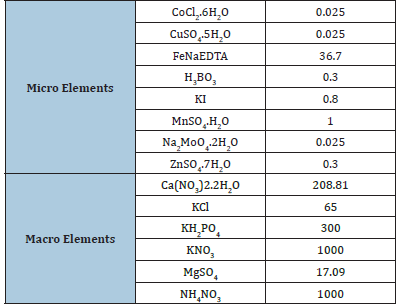
Two different trials were conducted. First, explants of C. alpina, P. cordatum and D. ruyschiana were collected in different times during august and september 2023 and placed in a Greesshoff and Doy (GD) medium (Table 3). While, in the second, the sterilization of explants of R. hypoglossum, H. officinalis and G. asclepiadea was conducted by applying or not PPM™ at the concentration of 2mlL-1 in addition to the protocolos described in Table 2. Explants were maintained at 24 °C with a photoperiod of 16h light/8h dark under 25-30μmolm-1 s-2 under cool, fluorescent white lamps. In both trials, after 35 days from the beginning of the in vitro experiment, the percentage of established explant, the percentage of shoot induction, the number and the lenght of shoot produced per plant, and the percentage of browning explants were measured.
Table 3:Micro and macro elements containing in the used micropropagation medium (Gresshoff and Doy medium;GD; mgL-1).
Statistical analysis
Significant differences were verified with the t-test (p < 0.05) after checking the data for normality (Shapiro-Wilk’s test, p ≥ 0.05) and homoscedasticity (Levene’s test, p ≥ 0.05). Moreover, a one-way ANOVA test by applying Ryan–Einot–Gabriel–Welsch Studentized Range Q (REGW-Q) post hoc test (p < 0.05) was performed to note differences between the collecting time of D. ruyschiana. These statistical analyses were computed by SPSS software (version 26.0, SPSS Inc., Chicago, IL, USA).
Results and Discussion
The anthropogenic load and the low competitiveness of the plant species in phytocenoses are the main reasons for the rarity of some plant species [7]. Moreover, these rare plant species are low seed germination or vegetative reproduction, relict species, torn areas, harsh climatic conditions, eaten by animals and birds [8]. In vitro culture protocols are widely used to solve these problems and to restore the gene pool of rare and endangered plant species. In the present study, for the first time, the importance of explant collecting times and the use of PPM in the first phases of micropropagation of Clematis alpina, Dracocephalum ruyschiana, Gentiana asclepiadea, Hyssopus officinalis, Phyteuma cordatum and Ruscus hypoglossum were tested.
The effect of different collecting times on in vitro development of C. alpina and D. ruyschiana explants are reported in Table 4. No parameters were affected in P. cordatum with 0.0 % of established explant. While, for both C. alpina and D. ruyschiana, collected explants had higher values in established explants and shoot induction were observed (Figure 1). Regarding the number of shoots produced per explant, an opposite dynamic was highlighted with a significant increase in C. alpina and a decrease in D. ruyschiana. In this last species was observed also a reduction in the percentage of browning. These findings indicated that, for the studied species, a later collection time in late august and September was most favourable because the mother plants are still in full activity with probably high levels of growth promoting substances and low growth inhibitors [9]. Furthermore, it was found that some genotypes responded well whereas, others are recalcitrant, which suggests that regeneration is genetically controlled. Regarding D. ruyschiana, low browning may be due to low in vivo phenolic content as the short-day length prevailing during this period has been reported to reduce the in vivo phenolics [10].
Figure 1:in vitro established explants of Clematis alpina (left) and Dracocephalum ruyschiana (right).

Table 4:Effect of different collecting times on the percentage of established explants (%), percentage of explants with shoot induction (%), number of shoots per explant (n.), mean lenght of shoot per explant (cm) and percentage of browning explants (%) of Clematis alpina, Phyteuma cordatum and Dracocephalum ruyschiana after 35 days from the beginning of the experiment.
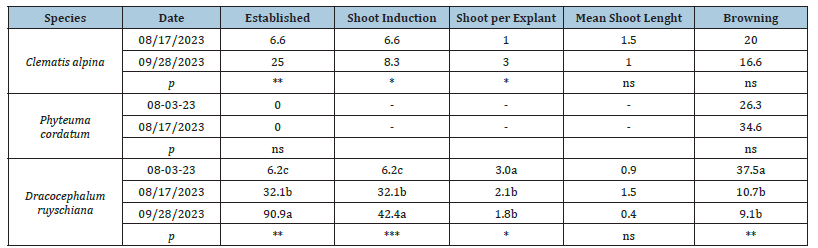
Note: Mean values showing the same letter are not statistically different at p < 0.05, according to the REGWF post hoc test. The statistical relevance is provided (* p < 0.05; ** p < 0.01; *** p < 0.001; ns = not significant).
The effective sterilization of biological material (e.g., initial explant) is required for successful in vitro culture initiation. Table 5 reports the effects of two sterilization protocols for micropropagation of R. hypoglossum and G. asclepiadea. Only R. hypoglossum explants were established in used GD medium. A significant reduction of this parameter was observed in the presence of PPM™. In the same medium an increase in browning was measured. Although no explants of G. asclepiadea was established, a reduction in browning was seen in the medium with PPM™. The data presented does not show a positive effect of the use of PPM™ in the sterilization protocols presented in this work. Likewise, Compton et al. [11] reported reduced embryo formation in Cucumis melo and poor shoot organogenesis of Petunia × hybrida even at low concentrations of PPM™. The use of PPM™ is generally limited to species that present contamination problems in the establishment stage such as Guadua angustifolia and Malus domestica [12]. Finally, as reported by Ledo et al. (2019) PPM™ has never been evaluated as a substitute for autoclaving. Apart from contamination, browning of excised plant tissues and nutrient media occurs frequently and remains a major basis for recalcitrance in vitro. The severity of browning has varied according to species, tissue or organ, developmental phase of plant, age of tissue or organ, nutrient medium and other tissue culture variables [13].
Table 5:Effect of sterilization with the presence or not of plant preservative mixture (PPM™) at the concentration of 2mlL-1 in addition to the protocolos described in Table 2, on the percentage of established explants (%), percentage of explants with shoot induction (%), number of shoots per explant (n.), mean length of shoot per explant (cm) and percentage of browning explants (%) of Ruscus hypoglossum, Hyssopus officinalis and Gentiana asclepiadea after 35 days from the beginning of the experiment.
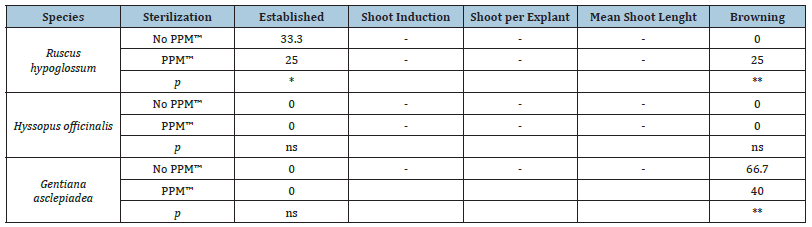
Note: The statistical relevance is provided (* p < 0.05; ** p < 0.01; ns = not significant).
Figure 2:Division of elongated shoots of Dracocephalum ruyschiana in aspetic conditions.

Conclusion
Up to date, there was no information regarding the micropropagation protocols for C. alpina, D. ruyschiana, G. asclepiadea, H. officinalis, P. cordatum and R. hypoglossum. In the present study, explant establishment and shoot multiplication technique was standardized mainly for D. ruyschiana (Figure 2).
Nodal segments explants proved to be useful for initiating micropropagation of this species. When the explants were collected during late summer season there was higher explant establishment and lower loss of the cultures due to explant browning and endogenic contamination. Regarding sterilization, the used protocols were not able to increase establishment in R. hypoglossum, H. officinalis and G. asclepiadea.
Taking all these considerations together, the direction of micropropagation research will enable a number of rare endangered species to be saved and in a number of cases to achieve a measure of success in ecological ex vitro repatriation of some species.
References
- Khlebova LP, Mironenko ON, Brovko ES (2021) In vitro micropropagation of wild rare plant Rhododendron ledebourii Pojark. IOP Conference Series: Earth and Environmental Science 723: 022033.
- Pace I, Chiavazza PM (2020) Low Germination Success in Phyteuma cordatum Balb and Empetrum hermaphroditum Biodiversity Online Journal 1(2).
- Yadav K, Singh N (2011) In vitro flowering of shoots regenerated from cultured nodal explants of Spilanthes acmella -an ornamental cum medicinal herb. Analele Universitatii din Oradea- Fascicula Biologie 18(1): 66-70.
- Bertsouklis K, Paraskevopoulou AT, Petraki E (2023) In vitro regeneration from adult node explants of Juniperus oxycedrus. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 51(1): 13062-13062.
- Srivastava V, Nerwal DK, Kandan A, Akhtar J, Sharma N, et al. (2021) Management of microbial contaminants in the in vitro Gene Bank: a case study of taro [Colocasia esculenta (L.) Schott]. In Vitro Cellular & Developmental Biology-Plant 57: 152-163.
- Niedz RP, Bausher MG (2002) Control of in vitro contamination of explants from greenhouse-and field-grown trees. In Vitro Cellular & Developmental Biology-Plant 38: 468-471.
- Chokheli VA, Dmitriev PA, Rajput VD, Bakulin SD, Azarov AS, et al. (2020) Recent development in micropropagation techniques for rare plant species. Plants 9(12): 1733.
- Caser M, Demasi S, Mozzanini E, Chiavazza PM, Scariot V (2022) Germination performances of 14 wildflowers screened for shaping urban landscapes in mountain areas. Sustainability 14(5): 2641.
- Silveira AAd, Lopes FJF, Sibov ST (2020) Micropropagation of Bambusa oldhamii Munro in heterotrophic, mixotrophic and photomixotrophic systems. Plant Cell Tiss Organ Cult 141: 315-326.
- Singh P, Patel RM (2016) Factors affecting in vitro degree of browning and culture establishment of pomegranate. African Journal of plant science 10(2): 43-49.
- Compton ME, Koch JM (2001) Influence of Plant Preservative Mixture (PPM) TM on adventitious organogenesis in melon, petunia, and tobacco. In Vitro Cellular & Developmental Biology-Plant 37: 259-261.
- Jiménez VM, Castillo J, Tavares E, Guevara E, Montiel M (2006) In vitro propagation of the neotropical giant bamboo (Guadua angustifolia) through axillary shoot proliferation. Plant Cell Tissue Organ Cult 86: 389-395.
- Huang LC, Lee YL, Huang BL, Kuo CI, Shaw JF (2002) High polyphenol oxidase activity and low titratable acidity in browning bamboo tissue culture. In Vitro Cellular & Developmental Biology-Plant 38: 358-365.
© 2023 Paola Maria Chiavazza. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






