- Submissions

Full Text
Modern Concepts & Developments in Agronomy
Biological Management of Euphorbia Helioscopia Weed Using Different Fungal Antagonistic Microorganisms
Muhammad Nasir Subhani* and Muhammad Ali
Department of Plant Pathology, Pakistan
*Corresponding author: Muhammad Nasir Subhani, Department of Plant Pathology, Faculty of Agricultural Sciences, Lahore, Pakistan
Submission: May 31, 2022;Published: June 15, 2022

ISSN 2637-7659Volume11 Issue 1
Abstract
Euphorbia helioscopia is commonly known weed of important crops. In addition to significant losses of the final yield this weed competes with crop population for nutrients and space. Thus, this problem needs a sustainable management approach in order to keep the environment and farmer health secure. Research trial was conducted to evaluate the inhibition potential of different fungal species (Alternaria tenuissimia, Alternaria alternate and Fusarium oxysporum) against E. helioscopia. Trial was conducted in March 2020 and designed with three treatments and five replicates. Seedlings were treated with different concentrations (1×103, 1×105, 1×107spores/ml.) of fungal spore suspensions of selected fungi. Experiment without any treatment was kept as control. Highest infection was recorded in the treatment of A. tenuissimia. Other developmental traits of the crop plants were also studied such as number of infected leaves, number of infected plants. Control experiment was remained un-infected.
Keywords: Euphorbia helioscopia; Fungal pathogens; Spore suspension, Sustainable agriculture
Introduction
Euphorbia helioscopia is also known as Sun spurge and belong to a vast family of euphorbiaceae. All the genus belongs to this family can be found in a number of climatic zones but most concentrated in humid tropics and subtropics Uzair M et al. [1]. In Pakistan, different Euphorbia species (e.g., E. granulata Forssk., E. helioscopia L., E. heterophylla L., Euphorbia hirta L., and E. prostrata J. Graham) are known as most notorious weeds of agricultural crops as well as vegetables and orchards Tanveer et al. [2]. With more than two thousand species, Euphorbia stood largest genus of Euphorbiaceae which consists of a botanical variety, ranging from shrubs to annual trees. Presence of latex and unique floral formula make this genus unique among this family (Barla A et al. [3], Chaudhry BA et al. [4] and Jassbi AR et al., 2006). In Pakistan Euphorbia helioscopia is known as a notorious weed of vegetable crops and responsible for a great loss in crop yield. Allelopathic influence of various weeds has been investigated on agricultural crops but not much research has been done on the allelopathic effect of Euphorbia helioscopia Mishra et al. [5]; Shukla et al. [6]; Kadioglue et al. [7] and Singh et al. [8].
In addition to its severe effects on cultivable lands this weed is also responsible for tumor, problems of digestive system, sterility and abortion if mistakenly consumed. It also causes severe skin irritation if come in contact with this weed (Ha et al. [9]; Rossoff [10]. Species of genus Euphorbia reported to have medicinal importance and used for a number of skin and other ailments. Moreover, the extract of these species is also used as insecticide Uzair M et al. [1]. Previously, cultural, mechanical as well as chemical strategies were in practice to control plant infections but with the passage of time chemical control became a major threat to environment and community health. Thus, application of biological control agents to control plant pathogens was introduced. As these methods was equally safe for health and environment so, it became widely popular and center of sustainable agricultural research (Blossey et al., 2002-04). Keeping in mind the rapidly increasing environmental deterioration and health issues due to chemical pesticide, there is need to introduce some biological means to control weeds Nasim et al. [11].
Material and Methods
Isolation of fungi
Fungal pathogens were isolated from the necrotic leaf tissues of E.helioscopia as well as from the roots and rhizospheric soil (A.alternata, F.oxysporum). In order to isolate from tissues direct plate method was applied. Infected portions from leaves, roots and stem were cut into small pieces of 5mm2 and surface sterilized with 1% NaOCl solution [12-15]. Surface sterilized infected plant portions were rinsed in water, dried on filter paper and inoculated on petri plate containing MEA as nutrient media and incubated at (25±2° C). In case of soil, the isolation was made by serial dilution method. Isolated fungi were purified and identified by studying their macroscopic and microscopic characters. Isolated fungal species were applied on Euphorbia helioscopia leaves by using different concentrations of spore suspension. Different concentrations used were 1×103, 1×105, 1×107spores/ml. Diluents used to prepare spore suspensions were distilled sterilized water and mustard oil [16].
Application of inoculum
Two methods of application were used to apply water suspension of biocontrol agents. In first method, prepared spore suspension was simply sprayed on the target weed. While in second case, weed leaves were gently rubbed with corrosive material like sandpaper before inoculation. Then water suspension was sprayed.
Oil suspension was applied by following the same course of action as used to apply the water suspension i.e., application of suspension before and after rubbing. Oil suspension was applied with camel hairbrush. Fungal suspensions were sprayed two to three times in a week. Sterile water was applied to control experiment. To provide maximum humidity, treated plants were kept under plastic cover for 24 hours. After removal of plastic covers the plants were regularly observed for 21 days [17].
Disease incidence was estimated by the proportion of diseased leaves (number of diseased leaves per total number of leaves) per plant and number of spots (pustule) on each leaf, 7 days after inoculation. The experiment was carried out in a completely randomized design with three replications per treatment. Each replication consisted of one pot containing one plant. Data regarding parameters of no. of diseased leaves/plant and no. of spots on each leaf was statistically analyzed by applying Duncan Multiple range test.
Result and Discussion
The selected fungal organisms were antagonistic to E. helioscopia. Application of these fungal pathogens showed variable results in each inoculation method and in each concentration. Parameters studied for disease development were infected leaves per plant and number of spots per leaf or infected area/leaf. Application of different concentrations (103,105,107spores/ml) of Alternaria tenussimia by rubbing and without rubbing showed following results.
Application of inoculum through water suspension
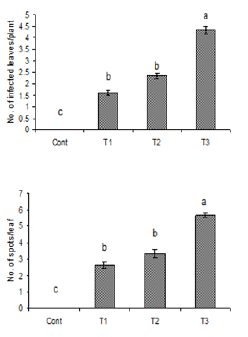
The statistics obtained from experiment showed variation in disease incidence and %age severity on E. helioscopia. Highest infection of E. helioscopia by A. tenussimia was with 45% incidence and 10% severity rating in case of water suspension without rubbing (Figures 1-3). Maximum no. of leaf spots (10 spots/ leaf) were observed at highest concentration i.e., 107spores/ml while minimum no. of leaf spots (4 spots/leaf) were observed at concentration 103spores/ml. The % age difference of disease development observed was 36%. Whereas by applying fungal spore suspension after gently rubbing the leaves surface, maximum infection observed was 33% at highest spore concentration (Figure 4).
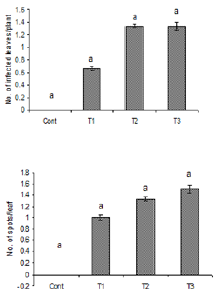
Figure 1: Number of infected leaves and spots per leaf
due to the inoculation of oil suspension of Alterneria
tenuissimia. Vertical bars show standard errors of
means of three replicates. Values with different letters
show significant difference (P=0.05) as determined by
DMR Test.
Control = water spray
T1= inoculated with 103 spores/ml
T2= inoculated with 105 spores/ml
T3= inoculated with 107 spores/ml
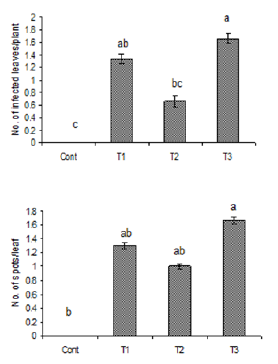
Figure 2: Number of infected leaves and spots per leaf
due to the inoculation of oil suspension of Alterneria
tenuissimia after rubbing. Vertical bars show standard
errors of means of three replicates. Values with
different letters show significant difference (P=0.05) as
determined by DMR Test.
Control = water spray
T1= inoculated with103 spores/ml
T2= inoculated with105 spores/ml
T3= inoculated with107 spores/ml
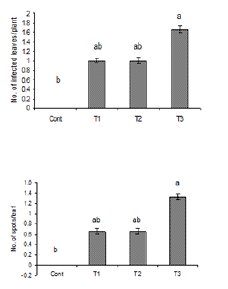
Figure 3: Number of infected leaves and spots per leaf
due to the inoculation of water suspension of Alterneria
tenuissimia. Vertical bars show standard errors of
means of three replicates. Values with different letters
show significant difference (P=0.05) as deter-mined by
DMR Test.
Control = water spray
T1= inoculated with103 spores/ml
T2= inoculated with105 spores/ml
T3= inoculated with107 spores/ml
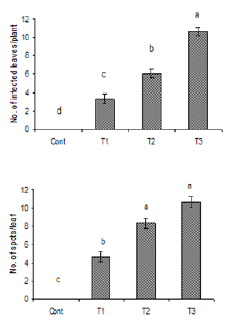
Figure 4: Number of infected leaves and spots per leaf
due to the inoculation of water suspension of Alterneria
tenuissimia after rubbing. Vertical bars show standard
errors of means of three replicates. Values with
different letters show significant difference (P=0.05) as
determined by DMR Test.
Control = water spray
T1= inoculated with103 spores/ml
T2= inoculated with105 spores/ml
T3= inoculated with107 spores/ml
Application of inoculum through oil suspension
Figure 5: Number of infected leaves and spots per leaf
due to the inoculation of oil suspension of Alterneria
alternate. Vertical bars show standard errors of means
of three replicates. Values with different letters show
significant difference (P=0.05) as determined by DMR
Test.
Control = water spray
T1= inoculated with103 spores/ml
T2= inoculated with105 spores/ml
T3= inoculated with107 spores/ml
A little bit infection was observed by applying inoculum through oil suspension. Maximum disease development was 7% and maximum no. of spots/leaf was 2%. These results were observed for plants without rubbing (Figure 1). After rubbing disease development was same as observed in plants without rubbing. While maximum no. of spots/leaf was 1%. Little infection in case of oil may be due to the closing of stomata or phytotoxic effects of oil [18] (Figures 5-7).
Figure 6: Number of infected leaves and spots per leaf due to the inoculation of oil suspension of Alterneria alternata
after rubbing. Vertical bars show standard errors of means of three replicates. Values with different letters show
significant difference (P=0.05) as determined by DMR Test.
Control = water spray
T1= inoculated with103 spores/ml
T2= inoculated with105 spores/ml
T3= inoculated with107 spores/ml
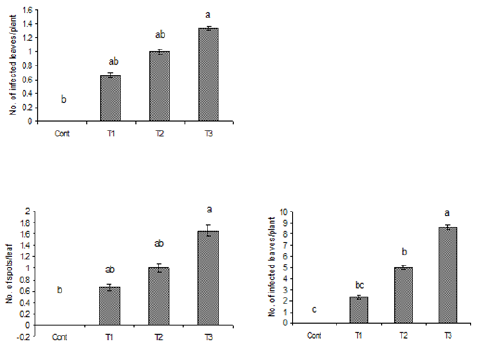
Figure 7: Number of infected leaves and spots per leaf due to the inoculation of water suspension of Alterneria
alternata. Vertical bars show standard errors of means of three replicates. Values with different letters show
significant difference (P=0.05) as determined by DMR Test.
Control = water spray
T1= inoculated with103 spores/ml
T2= inoculated with105 spores/ml
T3= inoculated with107 spores/ml
Application of Alternaria alternata on E. helioscopia
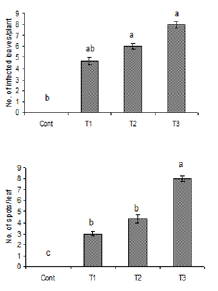
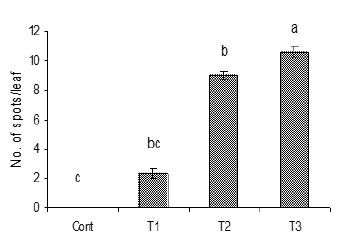
Maximum disease development was observed in plants that were inoculated after gently rubbing the leaves surfaces as compared to plants without rubbing. In case of rubbing, maximum disease development observed was 36% at highest concentration (Figure 8) while lowest infection observed was 10%. The %age difference calculated was 73.3%, whereas application of inoculum without rubbing the leaves surfaces showed highest infection 18% and lowest 7%. Maximum no. of spots/leaf calculated on plants was 10 after rubbing at higher concentration. Maximum infection was observed at higher concentration (107spores/ml) of A. alternata. The increase in incidence of disease on leaves with increasing inoculum concentration suggest that numbers of spores/conidia, which were retained then germinated and penetrated the tissues, probably increased with increasing inoculum density [19-21].
Figure 8: Number of infected leaves and spots per leaf
due to the inoculation of water suspension of Alternaria
alternata after rubbing. Vertical bars show standard
errors of means of three replicates. Values with
different letters show significant difference (P=0.05) as
determined by DMR Test.
Control = water spray
T1= inoculated with103 spores/ml
T2= inoculated with105 spores/ml
T3= inoculated with107 spores/ml
Application of inoculum through oil suspension
Minimum disease development was observed by oil suspension. Maximum no. of spots/leaf observed were two at 107spores/ml by rubbing and without rubbing. While highest infection observed was 6%. There was no difference in disease development by rubbing the plants, but spore concentration had slight effect on disease occurrence (Figures 5 & 6).
Application of F. oxysporum on E. helioscopia
Inoculation of F. oxysporum on soil around the E. helioscopia plants resulted in no infection. Spurge plants inoculated with this fungus have remained healthy. Absence of infection by inoculation of Fusarium oxysporum may be due to the saprophytic behavior of this fungal pathogen. Other reason may be the host specificity of pathogen [22-24].
Conclusion
This research work concluded that the spore suspension of Alternaria tenuissimia has significantly suppressed the growth of E. helioscopia.
Contribution
MA Designed the project and provided financial assistance. Also provided scientific assistance in performing trials and writeup. MNS: Co-supervisor provided research facilities and guidance.
References
- Uzair M, Loothar BA, Choudhary BA (2009) Biological screening of Euphorbia helioscopia L. Pak J Pharm Sci 22(2): 184-186.
- Tanveer A, Khaliq A, Javaid MM, Chaudhry MN, Awan I (2013) Implications of weeds of genus Euphorbia for crop production: a review. Planta Daninha 31(3): 723-731.
- Barla A, Biraman H, Kultur S, Oksuz S (2006) Secondary metabolites from Euphorbia helioscopia and their Vasodepressor activity. Turk J Chem 30(3): 325-332.
- Chaudhry BA, Janbaz KH, Uzair M (2001) Biological studies of Conyza and Euphorbia species. J Res Sci 12(1): 85-88.
- Mishra J, Swain SD, Singh VP (2004) Studies on germination and allelopathic potential of horse purslane (Trianthema partulacastrum L.). Indian J Plant Physiol 9: 181-184.
- Shukla AK, Prasad S, Srivastava SK, Singh SP, Singh RP (2003) Allelopathic effect of thatch grass (Imperata cylindrica L.) on various kharif and rabi season crops and weeds. Indian J Weed Sci 35(1,2): 163-166.
- Kadioglue I, Yanar Y, Asav U (2005) Allelopathic effects of weed leachates against seed germination of some plants. J Environ Biol 26(2): 169-173.
- Singh HP, Batish D, Shalinder K, Kohli RK, Dogra KS (2005) Allelopathic interference of Ageratum conyzoides L. against some crop plants. Weed management: balancing people, planet, profit 14th Australian Weeds Conference, Wagga, New South Wales, 6-9 September-2004, Papers and proceedings, Australia, pp. 558-561.
- Ha PK, Dongsoo K, Seungho L, Hoon L, Hoom K (2001) Anti-allergic and antiasthmatic activity of helioscopinin - A polyphenol compound, isolated from E helioscopia. J Microbiol Biotechnol 11(1): 138-142.
- Rossoff SI (2002) Encyclopedia of Clinical Toxicology:A Comprehensive Guide and Reference to the toxicology of prescription and OTC drugs, chemicals, herbals, plants, fungi, p. 445
- Nasim G, Shabbir A (2012) Invasive weed species-a threat to sustainable agriculture. In: Ashraf M, Öztürk M, et al. (Eds.), Crop production for agricultural improvement, Springer, Dordrecht, Netherlands, pp. 523-556.
- Basak SK, Bakshi PK, Basu S, Basak S (2009) Keratouveitis caused by Euphorbia plant sp. Indian J Ophthalmol 57(4): 311-313.
- Frohn A, Frohn C, Steuh KP, Thiel HJ (1993) Eye burns caused by wolfs milk. Ophtalmologe 90: 58-61.
- Frustenburger G, Hecker E (1985) On the active principles of the spurge family (Euphorbiaceae) XI (1). The skin irritant and tumor promoting diterpene esters of Euphorbia tricali L. originating from South Africa. Z Naturifrosch 40(9-10): 631-646.
- Giordani R, Trebaux J, Masi M, Regli P (2001) Enhanced antifungal activity of ketoconazole by Euphorbia characias latex against Candida albicans. J Ethnopharmacol 78(1): 1-5.
- Glattaar-Saalmuller B, Fallier-Becker P (2001) Antiviral action of Euphorbia com-positum and its components. Frosch Komplementamed Klass Naturheild 8(4): 207-212.
- Hussein G, Miyashiro H, Nakamura N, Kawahat Z, Otake T, et al. Z (1999) Inhibitory effects of Sudanese plant extraction on HLV-1 replication and HIV-1 protease. Phytother Res 13(1): 31-36.
- Johnson DA, Baudoin AM (1997) Mode of infection and factors affecting dis-ease incidence of loose smut of crabgrass. Biological Control 10(2): 92-97.
- Nasir E, Ali SI (1986) Flora of Pakistan, No.172, p.1. Panda H., 2004. Handbook of Medicina herb with uses. P.512.
- Natarujan D, Britto SJ, Srinivasan K, Nagamurugen N, Mohanasudari C, et al. (2005) Antibacterial activity of Euphorbia fusiformis. J Ethnopharmacol 102(1): 123126.
- Nawito M, Ahmed YF, Hecker E (1999) Dietary cancer risk from conditional cancerogens in produce of livestock fed on species of spruge (Euphorbiaceae). Dakk, Cairo, Egypt.
- Harris P, Paul HD, Schroeder D, Ronald V (1985) Biological control of leafy spurge in North America. Weed Science Society of America. Chapter 8 (3):79-92.
- Tanveer A, Rehman A, Javaid MM, Abbas RN, Sibtain M, et al. (2010) Allelopathic potential of Euphorbia helioscopia L. against wheat (Triticum aestivum L.), chickpea (Cicer arietinum L.) and lentil (Lens culinaris Medic.). Turk J Agric 34:75-81.
- Tilley AM, Walker HL (2002) Evaluation of Curvularia intermedia (Cochliobolus intermedius) as a potential microbial herbicide for large crabgrass (Digitaria sanguinalis). Biological Control 25(1): 12-21.
© 2022 Muhammad Nasir Subhani. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






