- Submissions

Full Text
Modern Approaches in Drug Designing
Emerging Diagnostic and Prognostic Biomarkers in Ovarian Cancer: A Review of Recent Advances
Moses Adondua Abah1*, Esther Edesiri Ajoku2, Okpanachi Nuhu Oyibo3, Nathan Rimamsanati Yohanna1, Joseph Oteng4, Naomi Ngozichukwuka Ajoniloju5,6, Jennifer Nnaemeka7, Macdonald Uchenna Eke8, Njideka Obioma Nwanne8, Rabiyya Halliru Abdullahi9 and Mary Omamomo Bosomo10
1Department of Biochemistry, Faculty of Biosciences, Federal University Wukari, Nigeria
2Healthcare Management and Administration, University of New Orleans, USA
3Department of Biochemistry, Faculty of Biological Sciences, University of Nigeria, Nigeria
4Ghana Health Service, Ghana
5Department of Pharmacology and Therapeutics, Faculty of Basic Clinical Sciences, Bowen University, Nigeria
6Department of Pharmacology and Therapeutics, Faculty of Basic Medical Sciences, University of Ibadan, Nigeria
7Department of Chemistry, Faculty of Arts and Sciences, Prairie View A&M University, USA
8Department of Medicine, College of Medicine, Imo State University, Nigeria
9Department of Community Medicine/Public health, Nile University of Nigeria, Nigeria
10Department of Physiology, Faculty of Basic Medical Sciences, University of Nigeria, Nigeria
*Corresponding author:Moses Adondua Abah, Department of Biochemistry, Faculty of Biosciences, Federal University Wukari, Nigeria
Submission: September 11, 2025;Published: October 13, 2025

ISSN: 2576-9170 Volume4 Issue 5
Abstract
Ovarian cancer is a leading cause of death among gynecological malignancies, with a high mortality rate due to late diagnosis and limited treatment options. The development of effective biomarkers for early detection and prognosis is crucial to improving patient outcomes. The integration of biomarkers into clinical practice has the potential to revolutionize ovarian cancer diagnosis and treatment. Emerging technologies, such as single-cell analysis and artificial intelligence, may enhance biomarker discovery and validation. Collaborative efforts are necessary to accelerate the development and clinical translation of biomarkers. The aim of this review is to explore the progress that has been made in diagnostic and prognostic biomarkers of ovarian cancer. Traditional biomarkers, such as Cancer Antigen 125 (CA- 125), and their limitations, as well as emerging biomarkers, including liquid biopsies, microRNAs, and proteomics were discussed in this review. Findings from this review revealed that ovarian cancer research has identified several emerging diagnostic and prognostic biomarkers. Ferroptosis-related genes, such as Kelch-like ECH-Associated Protein 1 (KEAP1), Interferon Gamma (IFNG), and Phosphorylase Kinase Catalytic Subunit Gamma 2 (PHKG2), are correlated with good prognosis and immune response. Circulating tumor DNA (ctDNA) is a promising biomarker for monitoring ovarian cancer recurrence and predicting treatment response. Interleukin-6 (IL-6) is associated with ovarian cancer diagnosis and prognosis, and may serve as a reliable diagnostic biomarker. Ataxia-Telangiectasia and Rad3-related kinase (ATR) and Phosphorylated ATR kinase (p-ATR) proteins are emerging as prognostic biomarkers and DNA damage response targets. Multi-marker panels, including CA125 and Human Epididymis Protein 4 (HE4), have shown improved sensitivity and specificity in diagnosing ovarian cancer. These biomarkers hold promise for improving diagnosis and treatment. The identification of emerging biomarkers in ovarian cancer holds promise for improving diagnosis, prognosis, and treatment. Recent advances in ferroptosis-related genes, ctDNA, IL-6, and ATR/p-ATR have potential clinical applications. Further research is needed to validate these findings and translate them into clinical practice effectively.
Keywords: Ovarian cancer; Biomarkers; Diagnosis; Prognosis; Artificial intelligence; Personalized medicine
Introduction
Ovarian cancer is a heterogeneous group of malignancies that originate primarily from the epithelial cells lining the ovary, fallopian tubes, or peritoneum. Histologically, over 95% of ovarian cancers are epithelial in origin, with high-grade serous carcinoma being the most prevalent and aggressive subtype [1]. Recent pathological insights suggest that many of these tumors actually arise from the distal fimbriae of the fallopian tubes rather than the ovarian surface epithelium, challenging traditional views of tumor origin [2]. This shift in understanding has significant implications for prevention strategies, such as prophylactic salpingectomy in high-risk women. The disease typically spreads via intraperitoneal dissemination, implanting on peritoneal surfaces such as the omentum and diaphragm, which contributes to its rapid progression and poor prognosis when diagnosed at advanced stages. Once peritoneal metastases develop, tumor cells can also induce ascites formation, further facilitating dissemination and impairing quality of life [3]. Globally, ovarian cancer affects over 300,000 women annually and accounts for more than 200,000 deaths, making it the most lethal gynecologic malignancy [4]. The absence of specific symptoms in early stages, coupled with the anatomical inaccessibility of the ovaries and lack of effective screening tools, results in delayed diagnosis for the majority of patients. Symptoms such as bloating, abdominal discomfort, and urinary urgency are often vague and mistaken for benign conditions, causing critical delays in clinical evaluation [5]. Consequently, most cases are detected at stage III or IV, where five-year survival rates plummet below 30%, despite aggressive cytoreductive surgery and combination chemotherapeutic regimens, often including platinum-based agents and targeted therapies [6].
In light of these challenges, the identification and application of biomarkers have emerged as a pivotal strategy in improving ovarian cancer outcomes. Biomarkers molecular indicators of physiological or pathological processes offer the potential to detect disease earlier, predict therapeutic response, and guide personalized treatment [7]. Despite their widespread usage, traditional markers like CA- 125 have poor sensitivity and specificity, especially in early-stage disease, and can be elevated in benign gynecological conditions. Consequently, reliance on a single biomarker is insufficient for accurate diagnosis or monitoring. Novel biomarkers, such as circulating tumor DNA, BRCA1/2 mutations, and Human Epididymis protein 4 (HE4), have been discovered owing to advances in genomics, proteomics, and metabolomics. These emerging candidates are being evaluated for their diagnostic, prognostic, and predictive value [8,9]. In hereditary ovarian cancer syndromes, genetic biomarkers play a dual role identifying high-risk individuals and guiding risk-reducing interventions. In addition to improving risk assessment and treatment planning, these molecular tools are central to the development of theranostic platforms that combine targeted imaging with precision therapeutics. Liquid biopsy technologies, capable of detecting tumor-derived components such as exosomes, microRNAs, and methylated DNA fragments in biofluids, offer a minimally invasive approach for serial monitoring [2]. Furthermore, multi-biomarker panels integrating genetic, proteomic, and metabolic signatures promise to enhance early detection and provide a more nuanced understanding of tumor heterogeneity and evolution.
This study aimed at providing an overview of current advancements in the detection and validation of diagnostic and prognostic biomarkers for ovarian cancer. The study considered the molecular landscape of the disease, evaluated the clinical usefulness of both existing and recently identified biomarkers, and investigated the technological platforms that have enabled these findings. Multi-omics techniques that combine proteomic, transcriptomic, epigenomic, and genomic data to uncover intricate biomarker networks influencing the development and spread of tumors will receive special attention. These integrated studies will support the development of precision medicine methods by identifying new therapeutic targets in addition to possible diagnostic tools. Moreover, the review will discuss existing constraints, including the necessity for strong validation studies, the difficulties in converting laboratory results into standard clinical practice, and the variation in biomarker performance across various groups. In order to improve prognostication, facilitate earlier detection, and aid in the development of more specialized and efficient treatment plans, this work attempts to critically analyze the literature in order to provide insight into potential future research directions. The incorporation of validated biomarkers into clinical processes has the potential to revolutionize ovarian cancer care, enhance patient survival, and drastically lower the global disease burden by facilitating a paradigm change from reactive treatment to proactive disease management.
Traditional Biomarkers in Ovarian Cancer
Among the earliest and most widely used biomarkers in ovarian cancer is Cancer Antigen 125 (CA-125), a high-molecular-weight glycoprotein encoded by the MUC16 gene. CA-125 is expressed on the surface of epithelial cells derived from the coelomic epithelium, including the ovaries, fallopian tubes, and peritoneum. Its elevation in serum is commonly associated with epithelial ovarian carcinoma, particularly in advanced stages [1]. Clinically, CA-125 has been employed for initial diagnostic evaluation, monitoring treatment response, and detecting recurrence. However, its utility is limited by poor sensitivity in early-stage disease and lack of specificity, as elevated levels may also occur in benign conditions such as endometriosis, pelvic inflammatory disease, menstruation, and pregnancy [10,11]. Consequently, CA-125 alone is insufficient for screening asymptomatic women or for definitive diagnosis, and its interpretation must be contextualized within imaging findings and clinical presentation. Despite these limitations, CA-125 remains a cornerstone in ovarian cancer management, particularly when used in serial measurements to assess therapeutic efficacy or disease progression.
To overcome the shortcomings of CA-125, other traditional biomarkers have been introduced, including Human Epididymis protein 4(HE4) and Cancer Antigen 72-4 (CA 72-4). HE4 is a secretory protein encoded by the WFDC2 gene and is frequently overexpressed in serous and endometrioid ovarian carcinomas. Unlike CA-125, HE4 levels are less influenced by menstrual cycle fluctuations or benign gynecologic conditions, making it a more specific marker for malignancy [12,13]. HE4 has demonstrated clinical utility in assessing the risk of malignancy in women with pelvic masses and is incorporated into algorithms such as the Risk of Ovarian Malignancy Algorithm (ROMA), which combines HE4 and CA-125 levels with menopausal status to improve diagnostic accuracy. Furthermore, HE4 is valuable in monitoring treatment response and detecting recurrence, often showing changes earlier than CA-125 alone. However, HE4 is not universally elevated in all ovarian cancer subtypes, particularly mucinous or germ cell tumors, limiting its role in those contexts.
This Figure 1 above illustrates the landscape of ovarian cancer biomarkers and diagnostic modalities [14].(A) Categorizes detection methods into four domains Serum, Tumor, Imaging, and Proximal Fluids highlighting traditional biomarkers like CA-125, HE4, and CA 72-4 alongside newer platforms such as ROMA®, OVA1®, and CancerSEEK. (B) Depicts molecular and cellular components involved in tumor profiling, including circulating tumor DNA (ctDNA), exosomes, autoantibodies, and genomic alterations such as methylation and aneuploidy.
Figure 1:Traditional and emerging biomarkers in ovarian cancer diagnostics. Source: Kinde et al. [14].
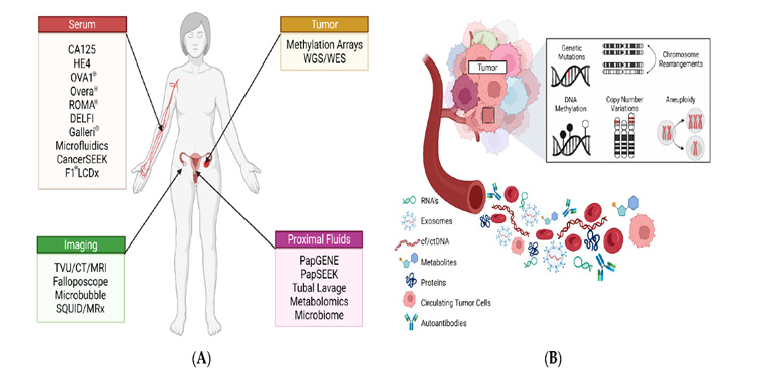
CA 72-4, another glycoprotein biomarker, is elevated in various adenocarcinomas, including gastric, breast, and ovarian cancers. In ovarian cancer, CA 72-4 has shown promise in complementing CA-125 and HE4, especially in cases where CA-125 is not elevated. Its levels are relatively stable across menstrual phases and pregnancy, enhancing its reliability in clinical settings. Studies have demonstrated that combining CA 72-4 with CA-125 and HE4 improves sensitivity and specificity in both diagnosis and follow-up of epithelial ovarian cancer [12]. Although CA 72-4 is less sensitive than CA-125 for detecting ovarian cancer on its own, its inclusion in multi-marker panels contributes to a more comprehensive assessment of disease status. Together, these traditional biomarkers form the foundation of ovarian cancer diagnostics, and their combined use continues to evolve as part of integrated strategies aimed at improving early detection and personalized care.
Emerging Diagnostic Biomarkers
The investigation of new diagnostic techniques that provide increased sensitivity, specificity, and non-invasive accessibility has been spurred by the shortcomings of conventional biomarkers in ovarian cancer [1]. Emerging biomarkers from liquid biopsies, microRNAs, and omics-based platforms like proteomics and metabolomics have been made possible by recent developments in molecular biology and bioinformatics. By analyzing circulating components in bodily fluids, liquid biopsy allows for real-time monitoring of tumor dynamics, marking a paradigm change in cancer diagnostics. Circulating Tumor Cells (CTCs), cell-free DNA (cfDNA), and exosomes are some of the most promising components [7].
Malignant cells known as CTCs are released into the bloodstream by primary or metastatic cancers. Their identification in ovarian cancer has been linked to a poor prognosis and the advancement of the illness. However, their therapeutic utility is still limited because of their scarcity and the technological difficulties in isolating and characterizing them [15]. However, improvements in immunoaffinity-based capture techniques and microfluidic technologies are raising the detection rates of CTCs and could increase their diagnostic use in the future. Tumor-specific genetic and epigenetic changes are carried by CfDNA, which is released following apoptosis, necrosis, or active secretion. By identifying mutations in TP53, BRCA1/2, and other oncogenes, cfDNA analysis has made it possible to identify tumor heterogeneity and therapy resistance in ovarian cancer [16]. Figure 2 presents a comprehensive overview of liquid biopsy as a transformative tool in the management of ovarian cancer, emphasizing its noninvasive nature and multi-dimensional utility across diagnosis, prognosis, and treatment monitoring. The diagram illustrates how tumors shed various biomolecules into the bloodstream including Circulating Tumor Cells (CTCs), cell-free DNA (cfDNA), exosomes, and circulating RNA (cfRNA) which can be captured through blood sampling and analyzed for clinically relevant insights. These components, derived from body fluids such as ascites, urine, and saliva, offer a dyna.
Figure 2:Liquid biopsy biomarkers in ovarian cancer. Source: Costa et al. [17].
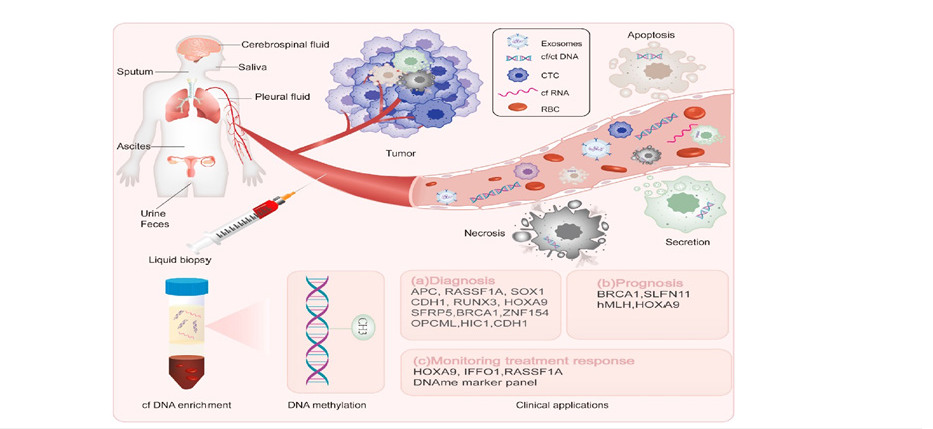
The integration of Oxford Nanopore and next-generation sequencing platforms has further enhanced the sensitivity of cfDNAbased diagnostics, making it a valuable tool for early detection and longitudinal monitoring. Exosomes Nano-sized extracellular vesicles secreted by tumor cells are rich in proteins, lipids, and nucleic acids that reflect the molecular landscape of the tumor. Their stability in circulation and accessibility from various fluids make them attractive candidates for biomarker discovery. Exosomal miRNAs and oncogenic proteins have demonstrated diagnostic and prognostic value in ovarian cancer, with studies showing their ability to differentiate malignant from benign conditions [17]. As isolation techniques become more refined, exosome profiling is expected to play a central role in personalized cancer diagnostics.
Short, non-coding RNAs known as microRNAs (miRNAs) control gene expression post-transcriptionally and are increasingly recognized for their role in ovarian cancer pathogenesis, including metastasis, apoptosis, proliferation, and chemoresistance. Their stability in body fluids such as blood, urine, and plasma makes them ideal candidates for non-invasive biomarker development. Figure 3 illustrates the complete miRNA biomarker pipeline, from biogenesis and extracellular release via exosomes and protein complexes, to extraction and quantification using qRT-PCR, microarrays, or sequencing. Clinically, miRNAs support diagnosis, prognosis, and personalized therapy, guiding treatment decisions and improving outcomes. For instance, miR-125b and miR-145 exhibit tumorsuppressive properties, while elevated levels of miR-200c, miR- 21, and miR-1290 correlate with poor prognosis [18]. Metaanalyses suggest that panels of circulating miRNAs outperform single markers, with some achieving sensitivity and specificity above 90% [19]. Recent studies employing machine-learning algorithms have further refined miRNA signature identification, enhancing predictive accuracy and facilitating clinical integration [20]. Nonetheless, challenges remain in standardizing detection platforms and validating findings across diverse populations.
Figure 3:MicroRNA biomarkers in ovarian cancer. Source: Ahn et al. [21].
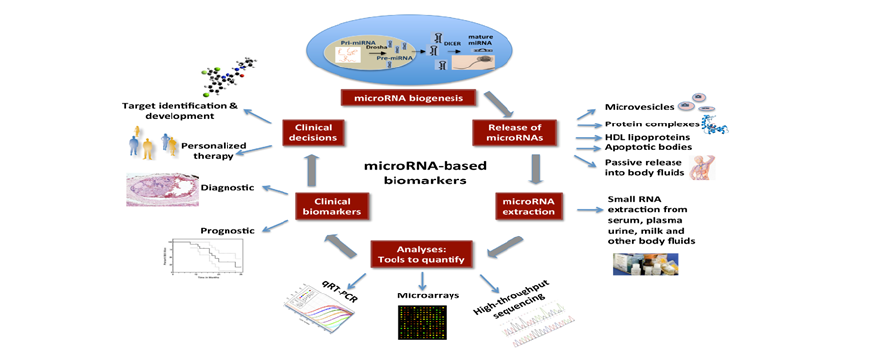
Comprehensive understanding of the molecular changes causing ovarian cancer can be gained through proteomics and metabolomics. Protein expression, changes, and interactions are analyzed on a broad scale in proteomics, whereas small-molecule metabolites that represent cellular physiology are the focus of metabolomics. Novel biomarker signatures have been discovered by recent integrative investigations using plasma proteome and metabolomic analysis. Tumor plasticity and metabolic reprogramming in high-grade serous ovarian cancer have been associated with proteins including PPCS, PMP2, and TUBB as well as metabolites like L-carnitine and phosphatidylcholine derivatives [21]. In addition to aiding in diagnosis, these biomarkers provide potential targets for therapeutic intervention. Metabolomics based on Extracellular Vesicles (EVs) has demonstrated special promise. EVs extracted from plasma show different metabolic signatures in patients with ovarian cancer than in healthy people and people with benign tumors. Area Under the Curve (AUC) values for machine learning models used to EV metabolite data have surpassed 0.90, indicating great diagnosis accuracy [22]. These results highlight how multi-omics integration might improve tailored care and early diagnosis.
Prognostic Biomarkers in Ovarian Cancer
One of the most important prognostic factors for ovarian cancer is genetic abnormalities, specifically in BRCA1 and BRCA2. These mutations affect treatment responsiveness and survival outcomes in addition to increasing the lifetime chance of contracting the disease. Due to impaired homologous recombination repair mechanisms, patients with BRCA mutations typically show increased sensitivity to platinum-based chemotherapy and benefit from PARP medicines. Apart from BRCA1/2, other high-risk variants have been shown to be emerging prognostic indicators, including RAD51C, RAD51D, and PALB2, which help with risk classification and individualized treatment plans [4].
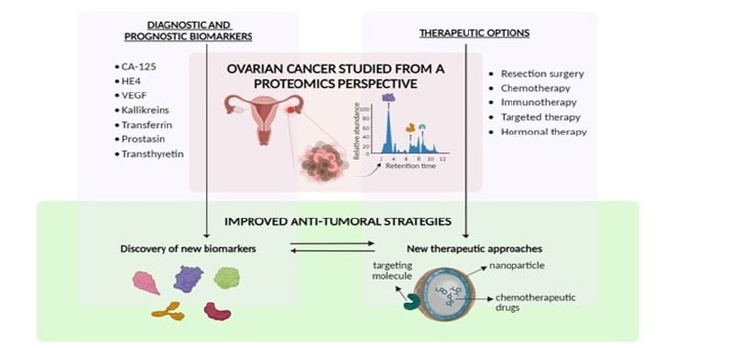
Figure 4 presents a proteomics-based framework for understanding and managing ovarian cancer, organized into three interconnected domains: diagnostic and prognostic biomarkers, therapeutic options, and improved anti-tumoral strategies. At the top, the figure highlights key protein biomarkers such as CA-125, HE4, VEGF, kallikreins, transferrin, prostatin, and transthyretin, which are instrumental in early detection, disease staging, and prognosis. These proteins, identified through high-throughput proteomic profiling, offer valuable insights into tumor biology and patient stratification. Gene expression profiling-derived molecular markers are now crucial instruments for forecasting ovarian cancer therapy response and overall survival. Clinicians can use these signals to categorize cancers into several molecular subgroups, each of which has a particular prognostic significance. For instance, immunosuppressive microenvironments or tumors with significant angiogenic activity are frequently associated with poor prognoses and resistance to conventional chemotherapy. These predictive models have been further improved by developments in multiomics technologies that integrate genomic, transcriptomic, and proteomic data, enabling prognostication that is more precise and customized treatment approaches [8]. In addition to molecular and genetic profiling, protein-based biomarkers like p53 and Ki-67 have significant predictive significance. Increased levels of Ki-67, a measure of cellular proliferation, are associated with aggressive tumor activity and lower survival rates. On the other hand, highgrade serous ovarian cancer and poor clinical outcomes are closely linked to abnormal p53 expression, which is frequently caused by TP53 mutations. Immunohistochemistry is frequently used to evaluate these biomarkers, which are crucial for directing treatment planning, staging, and grading. They improve predictive accuracy and aid in the development of targeted therapeutics when included in complete biomarker panels [4,8].
Figure 4:Ovarian cancer proteomics-driven understanding: biomarkers, treatments, and new approaches. Source: El Bairi et al. [4].
Genomic and Transcriptomic Biomarkers
Next-Generation Sequencing (NGS) has revolutionized the identification of genetic alterations in ovarian cancer, enabling high-throughput analysis of tumor genomes with unprecedented precision. Through NGS, researchers have uncovered recurrent mutations in genes such as TP53, BRCA1/2, PIK3CA, and KRAS, which are implicated in tumor initiation, progression, and therapeutic resistance. NGS also facilitates the detection of copy number variations, structural rearrangements, and epigenetic modifications, offering a comprehensive view of the tumor’s molecular landscape [1]. These insights have led to the development of targeted therapies and companion diagnostics, allowing clinicians to tailor treatment strategies based on individual genomic profiles [8]. As illustrated in Figure 5, the integration of genomic and transcriptomic biomarkers within a multi-omics framework enhances the precision of ovarian cancer management. Genomic tools such as Whole-Genome Sequencing (WGS) and Whole-Exome Sequencing (WES) enable the identification of hereditary and somatic mutations, including BRCA1/2 and TP53, which inform risk assessment and therapeutic decisions. Complementing this, transcriptomic approaches such as RNA-seq, single-cell RNA-seq (scRNA-seq), and spatial transcriptomics reveal dysregulated signaling pathways, immune evasion mechanisms, and cell-specific vulnerabilities that drive tumor heterogeneity and influence treatment response. Spatial transcriptomics, in particular, provides anatomical context to gene expression, guiding localized interventions. The figure underscores how these omics layers converge to support early diagnosis, patient stratification, and personalized therapeutic targeting, marking a paradigm shift toward precision oncology in ovarian cancer.
Figure 5:Integrated genomic and transcriptomic biomarkers in ovarian cancer. Source: El Bairi et al. [4].
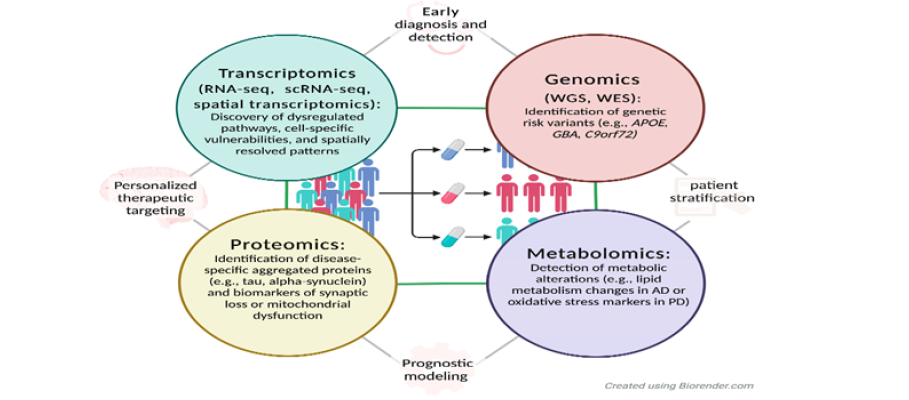
Gene expression profiling complements genomic analysis by revealing transcriptional changes that influence tumor behavior and treatment response. Using microarray and RNA sequencing technologies, researchers have identified distinct expression patterns associated with chemotherapy sensitivity, immune infiltration, and angiogenesis. For example, overexpression of genes involved in DNA repair or cell cycle regulation may predict resistance to platinum-based therapies, while immune-related gene signatures can indicate potential responsiveness to checkpoint inhibitors. These profiles not only serve as prognostic indicators but also help stratify patients for clinical trials and experimental therapies [4].
The integration of genomic and transcriptomic data has emerged as a powerful strategy for biomarker discovery in ovarian cancer. By combining mutational landscapes with gene expression dynamics, researchers can identify multi-dimensional biomarkers that offer greater predictive accuracy and biological relevance. This integrative approach enables the construction of molecular networks and pathway analyses that uncover novel therapeutic targets and resistance mechanisms. Moreover, multi-omics platforms are increasingly used to develop personalized treatment algorithms, improving clinical outcomes and minimizing toxicity. As computational tools and data repositories evolve, the potential for discovering robust, clinically actionable biomarkers continues to expand [4,8].
Epigenetic Biomarkers in Ovarian Cancer
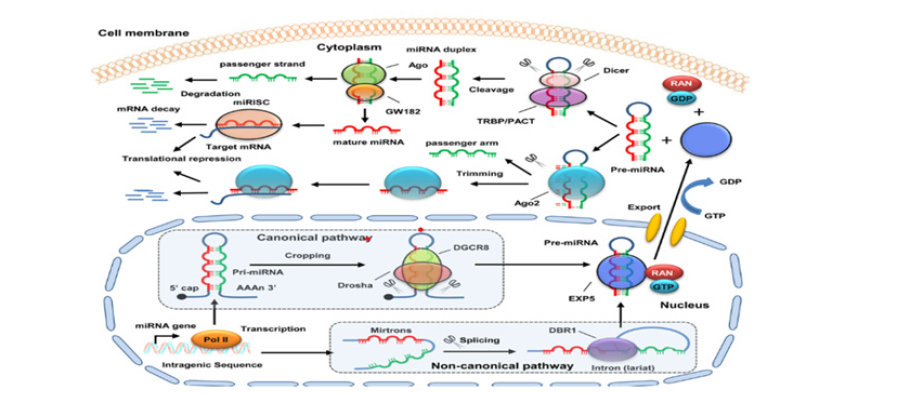
Despite being widely used, few putative biomarkers have been clinically validated. One major barrier that leads to diversity across investigations is the lack of standardized protocols for sample collection, processing, and analysis. Furthermore, biomarker performance must be carefully investigated in large, diverse patient populations to ensure consistency and therapeutic significance [1]. Cost-effectiveness remains a challenge, particularly in lowresource settings where access to advanced molecular diagnostics may be limited. López-Portugués et al. [23] stress the need for robust bioinformatics pipelines and standardized processes to overcome these challenges, while El Bairi et al. [4] draw attention to the ethical and practical difficulties of integrating multi-omics data into routine care. These complexities are vividly illustrated in the figure, which complements the discussion by mapping epigenetic signatures such as methylation and histone modifications alongside the biogenesis and function of microRNAs (miRNAs). The visual pathway of miRNA integration into the RNAinduced silencing complex (RISC), facilitated by Ago proteins and GW182, exemplifies the post-transcriptional regulation that often intersects with chromatin-level modifications. Just as histone methylation and acetylation patterns (like H3K27me3 or H3K9ac) shape gene accessibility, miRNAs act downstream to reinforce or disrupt expression profiles. Dysregulation in both layers histone modifications and miRNA activity contributes to the malignant transformation of ovarian cells, as represented by the coordinated export, processing, and targeting of miRNAs in the cytoplasmic domain of the diagram [7].
A crucial component of the epigenetic puzzle in the study of ovarian cancer, the figure (Figure 6: Biogenesis and Functional Pathways of MicroRNAs in Cellular Regulation) depicts the intracellular dynamics of microRNA maturation and function. The difficulties now outlined in sample handling, biomarker validation, and the application of molecular diagnostics across various patient populations are visually echoed by it. Biomarker consistency is hampered by the absence of defined procedures, as highlighted by López-Portugués et al. [23]. The canonical and non-canonical miRNA biogenesis pathways shown in the figure highlight the need for harmonization. These pathways rely on complex cellular machinery such as Drosha, DGCR8, Exportin 5, and Dicer. In situations with limited resources, these molecular players may contribute to diagnostic inconsistency if they are not accurately measured or interpreted.
Figure 6:Biogenesis and functional pathways of microRNAs in cellular regulation. Source: López-Portugués et al. [23].
Methylation signatures offer a promising avenue for individualized monitoring in ovarian cancer, as they can distinguish between benign and malignant lesions and potentially signal early disease recurrence. Epigenetic regulation through histone post-translational modifications such as acetylation, methylation, and phosphorylation plays a pivotal role in controlling gene expression and chromatin accessibility, and the dysregulation of histone-modifying enzymes like methyltransferases and Histone Deacetylases (HDACs) has been implicated in ovarian carcinogenesis [1]. Specific histone marks, including elevated H3K27me3 and altered H3K9ac levels, are associated with aggressive tumor phenotypes and poor clinical outcomes, as noted by López-Portugués et al. [23], and these modifications not only serve as biomarkers but also represent viable therapeutic targets, with HDAC inhibitors and other epigenetic drugs currently under clinical investigation. Their detectability in biofluids like blood and ascitic fluid positions these ncRNAs as compelling candidates for liquid biopsy-based diagnostics, and therapeutic strategies targeting them are being actively explored to restore normal gene expression and enhance treatment efficacy. Most remarkably, the accompanying figure visually reinforces the clinical relevance of miRNAs like let-7, miR-21, and miR-200c, whose regulatory loops are central to the molecular pathology of ovarian cancer and whose presence in biofluids underscores their diagnostic utility, thus bridging the biochemical mechanisms discussed with the broader epigenetic landscape of ovarian cancer [1]. It does this by showing the maturation These findings are supported by the discovery that non-coding RNAs (ncRNAs), including circular RNAs (circRNAs), long non-coding RNAs (lncRNAs), and microRNAs (miRNAs), are important regulators of gene expression in ovarian cancer. These ncRNAs affect immune evasion, chemoresistance, and the transition of epithelium to mesenchymal tissue. For example, dysregulated miRNAs like let-7, miR-200c, and miR-21, as well as lncRNAs like HOTAIR and MALAT1, modulate transcriptional activity and chromatin remodeling.
Clinical Applications and Challenges
The management of ovarian cancer has greatly improved with the introduction of molecular biomarkers into clinical practice, providing new instruments for prediction, diagnosis, and tailored treatment. However, a number of obstacles prevent their widespread implementation, even in the face of encouraging discoveries. The use of biomarkers to inform clinical judgment in ovarian cancer is growing [7]. Gene expression patterns aid in patient stratification according to anticipated chemotherapeutic response, and genomic changes like BRCA1/2 mutations are regularly tested to find candidates for PARP inhibitor therapy. Liquid biopsies are being investigated for the early identification of epigenetic indicators, such as DNA methylation patterns and non-coding RNA signals. According to López-Portugués et al. [23], proteomic and transcriptome biomarkers are becoming increasingly important for improving diagnostic precision and tracking the course of disease. Meanwhile, El Bairi et al. [4] highlight the utility of immune-related gene signatures and angiogenic markers in predicting response to immunotherapy and anti-angiogenic agents.
Few potential biomarkers have received clinical validation, despite their widespread use. The absence of established procedures for sample collection, processing, and analysis is a significant obstacle that causes variation amongst investigations. Furthermore, to guarantee consistency and therapeutic significance, biomarker performance needs to be thoroughly examined in sizable, varied patient cohorts. Cost-effectiveness is still an issue, especially in environments with low resources where access to sophisticated molecular diagnostics may be restricted. To get beyond these obstacles, López-Portugués et al. [23] emphasize the necessity of standardized procedures and strong bioinformatics pipelines. The logistical and ethical challenges of incorporating multi-omics data into standard care are also highlighted by El Bairi et al. [4].
To fully realize the potential of biomarkers, future efforts must focus on developing integrated diagnostic platforms that combine genomic, transcriptomic, epigenetic, and proteomic data. Artificial intelligence and machine learning algorithms are poised to play a key role in interpreting complex biomarker profiles and generating actionable insights. López-Portugués et al. [23] advocate for the creation of centralized biobanks and data-sharing networks to accelerate biomarker discovery and validation. Moreover, clinical trials should incorporate biomarker-driven stratification to optimize treatment outcomes and reduce unnecessary toxicity. As precision oncology continues to evolve, the integration of validated biomarkers into clinical workflows will be essential for improving survival and quality of life in ovarian cancer patients.
Future Perspectives and Opportunities
Intelligent data systems, collaborative innovation, and disruptive technology are shaping the future of ovarian cancer biomarker research. These advancements hold the potential to enhance long-term results, customize treatment, and improve diagnostics. By finding uncommon cell populations that promote metastasis and treatment resistance, single-cell analysis allows researchers to analyze tumor heterogeneity at a previously unheardof level of detail [24]. By maintaining tissue architecture, spatial transcriptomics provides an additional layer that makes it possible to trace gene expression inside the tumor microenvironment. These technologies are essential for finding context-specific biomarkers and comprehending cellular connections that affect the course of disease, according to Rajapaksha et al. [9]. It is anticipated that their incorporation into ovarian cancer research would enhance biomarker precision and uncover new treatment targets.
Advanced computational tools are necessary due to the complexity of multi-omics data. Algorithms for Machine Learning (ML) and Artificial Intelligence (AI) are being utilized more and more to stratify patients, predict treatment response, and find biomarker trends. Large datasets may be processed by these systems, which can also find hidden relationships and change over time [25]. Rajapaksha et al. [9] highlight the use of AI to speed up biomarker validation and facilitate real-time clinical decision-making. In order to improve patient outcomes, theranostic techniques that combine diagnostics and therapies are also supported by AI-driven platforms.
To close the translational gap between the discovery of biomarkers and clinical application, international cooperation is crucial. To guarantee reproducibility and scalability, Rajapaksha et al. [9] support centralized biobanks, multi-institutional collaborations, and standardized procedures. In order to validate predictive models and provide access to precision medicines, collaborative clinical trials that use biomarker-guided stratification are essential. The care of ovarian cancer can be revolutionized by accelerating innovation and bringing academic, clinical, and industry partners together.
Conclusion
Due in large part to late-stage detection and few available treatments, ovarian cancer continues to rank among the most deadly gynecologic cancers. This study emphasizes how genomic, transcriptomic, and epigenetic biomarkers have the ability to revolutionize ovarian cancer diagnosis and prognosis. The discovery of predicted transcriptional signatures and actionable genetic changes has been made possible by developments in nextgeneration sequencing and gene expression profiling. Histone alterations, non-coding RNAs, and DNA methylation patterns are examples of epigenetic biomarkers that provide extra layers of information on tumor biology and treatment resistance. Artificial intelligence and emerging technologies like single-cell analysis and spatial transcriptomics are speeding up the process of finding and validating biomarkers. To apply these discoveries in clinical practice, cooperation between disciplines and institutions is crucial.
The integration of validated biomarkers into clinical workflows holds promise for early detection, personalized treatment, and improved patient outcomes. Biomarker-guided stratification can optimize therapeutic choices, reduce toxicity, and enhance the efficacy of targeted and immunotherapies. However, challenges remain in standardizing methodologies, ensuring cost-effectiveness, and achieving regulatory approval. Future research must prioritize large-scale validation studies, harmonized protocols, and equitable access to molecular diagnostics.
Future research should concentrate on creating multi-omics platforms, liquid biopsy technologies, and AI-driven decision support systems in order to close the gap between discovery and implementation. These developments may make it possible for dynamic risk assessment, adaptive treatment plans, and realtime monitoring. The sector is well positioned to revolutionize ovarian cancer care by adopting new technologies and encouraging international cooperation, shifting from reactive treatment to proactive, precision-guided management.
References
- Victoria IA, Moses AA, Ale EM, Lyambee KQ, Victor O, et al. (2025) Effect of cucumis callosus fruit extract on the kidney function of DMBA-induced mammary cancer in female albino Wistar rats. Canc Therapy & Oncol Int J 28(4): 556245.
- Hassan M, Sonoda Y (2025) Ovarian cancer: Pathophysiology and etiology. Medscape.
- Saad A, Ameen OA, Ahmed FA, Moses AA (2025) Antidiabetic effect of chrysophyllum albidum pulp extract on streptozotocin-induced diabetic rats. Curre Res Diabetes & Obes J 18(1): 555976.
- Bairi EK, Kandhro AH, Gouri A, Mahfoud W, Louanjli N, et al. (2017) Emerging diagnostic, prognostic and therapeutic biomarkers for ovarian cancer. Cellular Oncology 40(2): 105-118.
- Anih DC, Arowora KA, Ugwuoke KC, Abah MA, Habibu B, et al. (2025) Nutritional modulation of epigenetic changes induced by mycotoxins: A biochemical perspective for at risk populations in africa. Science International 13(1): 90-109.
- Anih DC, Arowora AK, Abah MA, Ugwuoke KC (2025) Biochemical effects of microplastics on human health: A comprehensive review. Science International 13(1): 27-34.
- Ale EM, Kade IJ, Timothy MJ, Richard HNB, Steve OAS, et al. (2025) Assessment of the participation of sulfhydryl proteins in the glutathione peroxidase mimicry of diphenyl diselenide in the presence of thiol alkylating agent. Sci Rep 15(1): 17482.
- Atallah GA, Aziz ANH, Teik CK, Shafiee MN, Kampan NC (2021) New predictive biomarkers for ovarian cancer. Diagnostics 11(3): 465.
- Rajapaksha W, Khetan R, Johnson IRD, Blencowe A, Garg S, et al. (2024) Future theranostic strategies: Emerging ovarian cancer biomarkers to bridge the gap between diagnosis and treatment. Frontiers in Drug Delivery 4: 1339936.
- Charkhchi P, Cybulski C, Gronwald, J, Wong FO, Narod SA, et al. (2020) CA125 and ovarian cancer: A comprehensive review. Cancers 12(12): 3730.
- Najid M (2025) CA 125 blood test: Clinical significance, interpretation, and limitations. Cancer Biology Research.
- Granato T, Midulla C, Longo F, Colaprisca B, Frati L, et al. (2012) Role of HE4, CA72.4, and CA125 in monitoring ovarian cancer. Tumor Biology 33(5): 1335-1339.
- (2025) What is HE4 and its role in ovarian cancer? Biology Insights.
- Kinde (2023) Nature medicine.
- Karimi F, Azadbakht O, Veisi A, Sabaghan M, Owjfard M, et al. (2023) Liquid biopsy in ovarian cancer: Advantages and limitations for prognosis and diagnosis. Medical Oncology 40(9): 265.
- Si HQ, Wang P, Long F, Zhong W, Meng YD, et al. (2024) Cancer liquid biopsies by oxford nanopore technologies sequencing of cell-free DNA. Molecular Cancer 23(1): 265.
- Susana JN, Ines AB, Jose LC, Sonia AM (2019) Liquid biopsy beyond circulating tumor cells and cell-free DNA. Acta Cytologica 63(6): 479-488.
- Kozłowski M, Borzyszkowska D, Golara A, Lubikowski J, Cymbaluk PA (2025) The role of microRNA in the prognosis and diagnosis of ovarian cancer. International Journal of Molecular Sciences 26(7): 3413.
- Wang X, Kong D, Wang C, Ding X, Zhang L, et al. (2019) Circulating microRNAs as novel potential diagnostic biomarkers for ovarian cancer: A systematic review and updated meta-analysis. Journal of Ovarian Research 12(1): 24.
- Hamidi F, Gilani N, Belaghi RA, Yaghoobi H, Babaei E, et al. (2023) Identifying potential circulating miRNA biomarkers for the diagnosis and prediction of ovarian cancer using machine-learning approach. Frontiers in Digital Health 5: 1187578.
- Ahn HS, Yeom J, Yu J, Kwon YI, Kim JH, et al. (2020) Convergence of plasma metabolomics and proteomics analysis to discover signatures of high-grade serous ovarian cancer. Cancers 12(11): 3447.
- Long F, Pu X, Wang X, Ma D, Gao S, et al. (2025) A metabolic fingerprint of ovarian cancer: A novel diagnostic strategy employing plasma EV-based metabolomics and machine learning algorithms. Journal of Ovarian Research 18(1): 26.
- López PC, Bayón MM, Díez P (2024) Biomarkers in ovarian cancer: Towards personalized medicine. Proteomes 12(1): 8.
- Moses AA (2025) Effect of green synthesized silver nanoparticles from orange peels on growth performance and liver function parameters of male albino rats. PriMera Scientific Surgical Research and Practice 5(5): 03-14.
- David CA, Kayode AA, Moses AA, Kenneth CU, Bilyaminu H (2025) Redefining biomolecular frontiers: The impact of artificial intelligence in biochemistry and medicine. Journal of Medical Sciences 25(1): 1-10.
© 2025 Moses Adondua Abah. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






