- Submissions

Full Text
Modern Approaches in Drug Designing
Thermosensitive Bioadhesive Gel for Periocular Herpetic Lesions: A Mini-Review and Perspective on Polymer-Based Strategies
Andreza Maria Ribeiro1*, Thais H S Flores-Sahaguns2 and Fabio Furtado2
1 Post-graduation Program in Department of Engineering and Material Sciences (PIPE), University of Federal of Parana (UFPR), Brazil
2 Department of Mechanical Engineering, University of Federal of Parana (UFPR), Brazil
*Corresponding author:Andreza Maria Ribeiro, Post-graduation Program in Department of Engineering and Material Sciences (PIPE), University of Federal of Parana (UFPR), Brazil
Submission: September 09, 2025;Published: October 09, 2025

ISSN: 2576-9170 Volume4 Issue 5
Abstract
Herpes Simplex Virus type 1 (HSV-1) remains a leading cause of recurrent periocular infections, often resulting in painful lesions, inflammation, and potential vision impairment. Conventional topical treatments, such as creams and ointments, offer limited retention and poor patient adherence, particularly in the delicate and mobile skin surrounding the eyes. This mini-review explores thermosensitive bioadhesive gel as a coadjuvant platform, designed to synergize with natural and synthetic polymers to improve therapeutic performance and user tolerability. The proposed formulation centers on Pluronic F127, a triblock copolymer known for its reversible thermal gelation, low toxicity, and high drug solubilization capacity. When combined with bioadhesive natural polymers such as xanthan gum, alginate, pectin, or chitosan, the gel forms a stable matrix capable of adhering to periocular skin, ensuring prolonged residence time and sustained drug release. In addition to conventional antivirals like acyclovir and ganciclovir, newer agents such as foscarnet, effective against acyclovir-resistant HSV strains, are considered for incorporation into advanced formulations. Expert Perspective: The integration of thermoresponsive and bioadhesive polymers offers a promising route to overcome the limitations of current topical therapies, especially in sensitive anatomical regions. These hybrid systems may redefine the management of periocular viral lesions by combining pharmacological efficacy with enhanced patient experience.
Keywords: Periocular herpetic lesions; Thermal polymers; Pluronic; Natural polymers; Bioadhesive gel; Antivirals
Mini Review and Perspective
Herpes simplex virus type 1 (HSV-1) is a leading cause of recurrent ocular and periocular infections, often resulting in painful lesions and long-term complications [1,2]. Conventional topical treatments, such as creams and ointments, offer limited retention and poor patient adherence, especially in the sensitive periocular region. This mini review explores the development of the formulations of a thermosensitive bioadhesive gel designed specifically for application on the skin surrounding the eyes. According to the World Health Organization, approximately 3.7 billion people under the age of 50 are infected globally with Herpes Simplex Virus type 1 (HSV-1), widely recognized for causing oral infections. Additionally, HSV-2 is responsible for genital herpes, which is transmitted through oral- genital contact [3,4]. It is important to highlight that HSV-1 affects newborns, infants, and adults, and can be transmitted through direct contact with infected secretions from symptomatic or asymptomatic hosts. Although 80% of infections are asymptomatic, they can occasionally lead to life- threatening complications. In symptomatic cases, HSV-1 typically produces vesicles or ulcers around the mouth, often preceded by sensations of tingling or burning in the affected area [1-4].
Expert Insight: The high prevalence and latent nature of HSV- 1 underscore the urgent need for innovative therapeutic strategies that go beyond symptomatic relief and target viral persistence and recurrence.
HSV-1 is also the leading cause of ocular herpes [5], which is frequently misdiagnosed as conjunctivitis, herpes zoster, Acanthamoeba infection, or drug-induced toxicity [4]. Typically, ocular infection occurs when a person touches a cold sore and then rubs their eyes with contaminated fingers. Once acquired, the virus remains in the body for life. Following an initial episode of ocular herpes [5], there is a 27% risk of recurrence within one year, 50% within five years, and 63% within twenty years, according to the American Academy of Ophthalmology [6].
Expert Insight: These recurrence rates highlight the chronic nature of ocular herpes and the limitations of current antiviral therapies, which often fail to prevent long-term complications or viral reactivation.
Ocular herpes tends to infect the cornea, causing symptoms such as eye inflammation, swollen lymph nodes near the eye, redness, tearing, light sensitivity, headache, blurred vision, and, in severe cases, vision loss [1,2]. Generally, only one eye is affected. Moreover, the virus may remain latent in the nervous system without causing symptoms. Herpes can lead to corneal ulcers and scarring, which may result in permanent vision loss [5,6]. Living with ocular herpes can have a significant emotional impact. The fear of vision loss and the possibility of recurrent outbreaks can severely affect the quality of life of those affected. HSV-1 is a DNA virus from the Herpesviridae family, with humans as its only host. Recurrent herpes primarily manifests as dendritic keratitis and, in rare cases, may be accompanied by conjunctivitis and eyelid vesicles [5,7].
Expert Insight: The psychosocial burden of ocular herpes is often underestimated. A truly effective treatment must address not only the virological and immunological aspects but also the emotional and functional well-being of patients.
Herpes zoster, often known as shingles, emerges when the dormant Varicella-Zoster Virus (VZV) reactivates after a primary infection. When this reactivation affects the ophthalmic branch of the trigeminal nerve, it manifests as ophthalmic herpes zoster, a condition that can severely compromise ocular health [5,7]. Among antiviral agents, acyclovir remains the gold standard. Its targeted action against HSV-1, HSV- 2, VZV, and even cytomegalovirus has made it the most widely prescribed molecule in recent decades. Its high specificity and low toxicity profile make it a reliable choice for both systemic and topical applications [8,9].
Expert Insight: Despite its clinical success, acyclovir’s limited penetration into ocular tissues and short retention time on the eye surface highlight the need for smarter delivery platforms.
Herpes simplex-induced keratitis, whether epithelial or stromal, is one of the most common opportunistic eye infections and a leading cause of vision loss worldwide. Topical acyclovir is frequently used to manage these cases, but its therapeutic window is narrow, and recurrence remains a challenge [8,9]. Ganciclovir, another potent antiviral, offers broader coverage across the herpesvirus family and is particularly effective in treating herpetic keratitis [9,10].
Expert Insight: Ganciclovir’s broader spectrum is promising, but its clinical efficacy hinges on sustained contact with the corneal surface-something conventional formulations struggle to achieve.
Foscarnet (phosphonoformic acid) is a pyrophosphate analog that directly inhibits viral DNA polymerase, making it effective against HSV strains resistant to acyclovir and ganciclovir. Unlike nucleoside analogs, foscarnet does not require activation by viral kinases, which allows it to bypass common resistance mechanisms. Although traditionally administered intravenously, recent studies have explored its topical use in ophthalmology. Awh et al. [11] reported successful treatment of six patients with refractory herpetic keratitis using foscarnet 2.4% eye drops, after failure with standard antivirals. The study demonstrated clinical improvement and tolerability, suggesting that foscarnet may be a viable option for localized therapy in resistant cases [11].
Expert Insight: Foscarnet’s unique mechanism of action and efficacy in resistant HSV infections make it a strategic candidate for incorporation into periocular formulations. This suggests potential for localized treatment in resistant cases, especially in periocular lesions where systemic therapy may be insufficient.
To overcome these limitations, researchers are turning to mucoadhesive systems, particularly hydrogels. Unlike traditional ointments and creams, these gel-based dressings adhere to the mucosal surface of the eye, allowing for prolonged drug residence and improved bioavailability [12]. The backbone of these systems lies in polymer science. Gel-forming polymers, when dispersed in water, create viscous matrices that can encapsulate and release drugs in a controlled manner [13,14]. The term “polymer” itself comes from the Greek polys (many) and meros (parts), reflecting their structure as long chains of repeating molecular units. These macromolecules have become essential components in pharmaceutical gel formulations [15]. Hydrogels, in particular, are three-dimensional networks of hydrophilic polymers capable of absorbing large volumes of water or biological fluids. Their insolubility, due to chemical or physical crosslinking, makes them stable carriers for therapeutic agents. Their compatibility with aqueous environments allows them to swell and adapt to biological tissues, making them ideal for medical applications [16-18].
Expert Insight: Hydrogels are more than passive carriers; they’re dynamic systems that can respond to environmental stimuli like temperature, making them especially attractive for ocular therapies where comfort and precision are key.
In recent years, hydrogels have shown great promise in transdermal and transmucosal drug delivery, offering controlled release and enhanced patient compliance [19,20]. For topical applications, bioadhesive formulations must exhibit optimal rheological and mechanical properties, such as pseudoplastic or plastic flow behavior with thixotropy, ease of application, good spreadability, appropriate hardness, and prolonged residence time on the skin. These characteristics directly influence the therapeutic performance and patient acceptance of the final product [20-24].
Expert Insight: In ocular drug delivery, patient comfort and formulation stability are not treats; they are prerequisites. A poorly tolerated gel, no matter how pharmacologically potent, will fail in real- world settings.
The excipients used in these vehicles vary widely, but they share colloidal properties that, upon contact with water, form gels of varying thickness and consistency. These networks allow for the incorporation of active substances within their polymeric mesh. Gels are generally classified as hydrophobic (oleogels) or hydrophilic (hydrogels). Hydrophilic gels use water or glycols, such as glycerin or propylene glycol, as their base. These liquids are gelled using agents like tragacanth gum, karaya gum, starch, cellulose derivatives, aluminum and magnesium silicates, pectins, alginates, carbomers, polyvinyl alcohol, among others [15,25].
Hydrophilic gels are the most commonly used due to their emollient properties. However, they tend to dry quickly, often forming brittle films on the skin [26]. Their excipients, composed of large colloidal molecules, cannot penetrate intact epidermis and lack affinity for skin proteins, resulting in minimal biochemical absorption.
Expert Insight: The challenge lies in balancing bioadhesion with permeability. A gel that sticks but doesn’t deliver the drug is no better than one that evaporates too quickly.
Some gels, especially those containing poloxamers (Pluronic) polymers, can be crosslinked to form therapeutic films or patches. The type of polymer used in the formulation significantly influences the gel’s rheological behavior, physical stability, and even its acceptability to patients [27,28]. Pluronics are surfactants classified by numerical codes that correlate with their molecular weight. For example, Poloxamer 101 (MW 1100) has a Hydrophilic-Lipophilic Balance (HLB) of 12-18, while Poloxamer 188 (MW 8400) has an HLB greater than 24. These compounds are minimally toxic and non-irritating to skin and eyes. They form gels with defined water concentrations:
a. Poloxamers 184 and 188: 50-60% water
b. Poloxamers 234 and 238: 40% water
c. Poloxamers 333-338: 25% water
Pluronic F127 is a triblock copolymer composed of poly (ethylene oxide)-poly (propylene oxide)- poly (ethylene oxide) PEO-PPO-PEO, with approximately 70% ethylene oxide units. It forms a clear gel in aqueous solutions at concentrations around 20% (w/w) or higher and exhibits a unique thermoreversible gelation behavior, solidifying at body temperature and reverting to a low-viscosity solution at refrigeration temperatures [20].
Expert Insight: Thermoreversible gels like Pluronic F127 gel offer a compelling advantage: they can be applied as liquids and transform into gels in situ, enhancing retention and minimizing discomfort.
Pluronic F127 is known for its low toxicity, high solubilizing capacity, and excellent drug release characteristics [28,29]. These PEO-PPO copolymers have been widely used as excipients in semi- solid pharmaceutical formulations [12,20,29]. Structurally, they consist of a hydrophobic polypropylene oxide core flanked by hydrophilic polyethylene oxide chains. Their general chemical formula is: HO(C₂H₄O)ₐ(C₃H₆O)ᵦ(C₂H₄O)ₐH, where “a” (ethylene oxide) ranges from 2 to 130 and “b” (propylene oxide) from 15 to 67.
Preparing Pluronic F127-based gels is straightforward. Cold preparation is preferred, as it facilitates dissolution and minimizes degradation. The polymer is mixed at 4-5 °C with other components (e.g., active drug) and cold water until a homogeneous solution is obtained [28,29].
Expert Insight: Cold processing not only preserves the integrity of sensitive drugs but also enhances reproducibility, a key factor in scaling up for clinical use.
Given the proximity to ocular tissues, ensuring sterility of the formulation is essential. Pluronic F127 can be sterilized using techniques such as steam heat, dry heat, and irradiation methods, including gamma and electron beam (e-beam) sterilization. Among these, e-beam irradiation at doses between 15-25kGy has been shown to preserve the hydrogel’s structural integrity and gelling properties, making it the most suitable method for ophthalmic applications [30,31].
Expert Insight: In periocular drug delivery, sterility is nonnegotiable. Selecting a sterilization method that maintains the functional properties of Pluronic-based hydrogels, without compromising safety or efficacy, is critical for clinical success.
On the other hand, natural polymers have gained increasing attention in topical drug delivery systems due to their biocompatibility, biodegradability, and minimal toxicity. In the context of periocular bioadhesive gels, several natural polymers can be incorporated to enhance mucoadhesion, rheological behavior, and drug permeation [15]. Xanthan gum, a polysaccharide produced by Xanthomonas campestris, is particularly promising. It exhibits excellent viscosity-building properties, shear-thinning behavior, and strong bioadhesive capacity, making it suitable for sensitive areas such as the eyelids and surrounding skin [32]. Other candidates include alginate (derived from brown algae), which forms hydrogels in the presence of divalent cations and offers soothing, film-forming properties; pectin, known for its gelling ability and compatibility with mucosal tissues; and chitosan, a cationic polymer obtained from chitin, which enhances drug permeation and possesses intrinsic antimicrobial activity [33,34]. These natural polymers can be used alone or in combination with synthetic agents like Pluronic F127 to create hybrid systems that balance mechanical stability with biological performance [28,35].
Expert Insight: The strategic inclusion of natural polymers not only could improve the bioadhesive and rheological properties of periocular gels, but also aligns with the growing demand for biocompatible and sustainable pharmaceutical systems, especially in formulations targeting delicate anatomical regions.
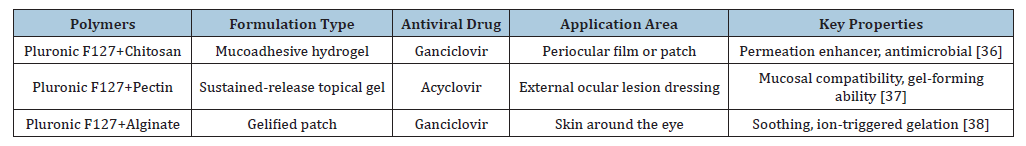
Antiviral drugs’ inclusion in advanced topical formulations could expand the therapeutic arsenal for managing herpetic lesions in sensitive facial regions, where conventional treatments often fall short [36-38] (Table 1).
Table 1:Overview of antiviral formulations and applications of Pluronic F127 and natural polymers.
Perspective
The development of thermosensitive bioadhesive gels for periocular application represents a promising strategy in the management of herpetic lesions. These formulations, particularly those based on Pluronic F127, offer unique advantages such as reversible gelation at physiological temperatures, prolonged adhesion, and controlled drug release. However, the therapeutic potential of these systems can be significantly enhanced through the incorporation of natural polymers as coadjuvants.
Polymers such as xanthan gum, alginate, pectin, and chitosan bring additional functional benefits, ranging from improved mucoadhesion and biocompatibility to enhanced rheological behavior and permeability. Their colloidal nature allows them to form stable networks that interact favorably with the epidermis, especially in sensitive regions like the eyelids and surrounding skin. When combined with synthetic agents like Pluronic F127, these natural polymers contribute to hybrid systems that are both structurally robust and biologically responsive. The synergy between synthetic and natural polymers is not merely a formulation choice; it is a therapeutic necessity. In periocular drug delivery, where precision, tolerability, and patient adherence are paramount, such combinations offer a balanced solution that meets clinical and practical demands. Looking ahead, the integration of multifunctional natural polymers into thermosensitive gels opens new avenues for personalized and sustainable treatments. These systems have the potential to redefine topical antiviral therapy, offering not only pharmacological efficacy but also enhanced patient experience and long-term safety.
In addition to conventional antivirals like acyclovir and ganciclovir, the incorporation of foscarnet, a pyrophosphate analog effective against acyclovir-resistant HSV strains, adds a valuable dimension to these formulations. Recent evidence supports its topical use in refractory herpetic keratitis, suggesting that its inclusion in bioadhesive gels could offer targeted efficacy with reduced systemic toxicity [11]. Foscarnet’s direct inhibition of viral DNA polymerase makes it a strategic candidate for periocular applications, especially when formulated within thermoresponsive systems that ensure localized delivery and sustained therapeutic action. Rheological and mechanical properties should be optimized to achieve pseudoplastic behavior, spreadability, and ease of application. The inclusion of complementary polymers enhances the gel’s structural integrity, permeability, and compatibility with the delicate periocular region. This coadjuvant strategy addresses key limitations in current topical therapies and offers a patientfriendly alternative for managing viral lesions.
The results suggest that thermosensitive bioadhesive gels, when formulated with synergistic polymer systems and advanced antiviral agents like foscarnet, could become a valuable tool in ophthalmic and dermatological care, combining pharmacological effectiveness with enhanced tolerability, precision, and adherence.
Acknowledgments
This work was supported by the Brazilian National Research Council, CNPq, Brazil. The authors wish to thank the National Council for Scientific and Technological Research (CNPq, fellowships process numbers 249251/2013-2 and 302459/2023-5).
Conflicts of Interest
The authors have declared no conflict of interest.
References
- Whitley RJ, Kimberlin DW, Roizman B (1998) Herpes simplex viruses. Clinical Infectious Diseases 26(3): 541-553.
- Valerio GS, Lin CC (2019) Ocular manifestations of herpes simplex virus. Current Opinion in Ophthalmology 30(6): 525-531.
- Gilden DH, Mahalingam R, Cohrs RJ, Tyler KL (2007) Herpesvirus infections of the nervous system. Nature Clinical Practice Neurology 3(2): 82-94.
- Bharucha T, Houlihan CF, Breuer J (2019) Herpesvirus infections of the central nervous system. Semin Neurol 39(9): 369-382.
- Marsh RJ, Cooper M (1993) Ophthalmic herpes zoster. Eye 7(3): 350-370.
- Liesegang TJ (2008) Herpes zoster ophthalmicus: Natural history, risk factors, clinical presentation, and morbidity. Ophthalmology 115(2 Suppl): S3-S12.
- Vrcek I, Choudhury E, Durairaj V (2017) Herpes zoster ophthalmicus: A review for the internist. The American journal of medicine 130(1): 21-26.
- Opstelten W, Eekhof J, Neven AK, Theo V (2008) Treatment of herpes zoster. Canadian Family Physician 54(3): 373-377.
- Clercq ED (1993) Antivirals for the treatment of herpesvirus infections. Journal of Antimicrobial Chemotherapy 32(Suppl_A): 121-132.
- Colin J, Hoh HB, Easty DL, Herbort CP, Resnikoff S, et al. (1997) Ganciclovir ophthalmic gel (Virgan; 0.15%) in the treatment of herpes simplex keratitis. Cornea 16(4): 393-399.
- Awh CC, Austen NK, Jeffrey MG, See CW, Lowder CY, et al. (2024) Foscarnet eyedrops for the treatment of refractory herpetic keratitis. Journal of Ophthalmic Inflammation and Infection 14(1): 54.
- Russo E, Villa C (2019) Poloxamer hydrogels for biomedical applications. Pharmaceutics 11(12): 671.
- Van DVK, Kiekens P (2002) Biopolymers: Overview of several properties and consequences on their applications. Polymer Testing 21(4): 433-442.
- Gao Y, Zhang X, Zhou H (2023) Biomimetic hydrogel applications and challenges in bone, cartilage, and nerve repair. Pharmaceutics 15(10): 2405.
- Satchanska G, Davidova S, Petrov PD (2024) Natural and synthetic polymers for biomedical and environmental applications. Polymers 16(8): 1159.
- Gupta P, Vermani K, Garg S (2002) Hydrogels: From controlled release to pH-responsive drug delivery. Drug discovery today 7(10): 569-579.
- Peppas NA, Bures P, Leobandung W, Ichikawa H (2000) Hydrogels in pharmaceutical formulations. European Journal of Pharmaceutics and Biopharmaceutics 50(1): 27-46.
- Ribeiro AM, Flores-Sahagun THS (2020) Application of stimulus-sensitive polymers in wound healing formulation. International Journal of Polymeric Materials and Polymeric Biomaterials 69(15): 979-989.
- Liechty WB, David RK, Brandon VS, Nicholas AP (2010) Polymers for drug delivery systems. Annual Review of Chemical and Biomolecular Engineering 1: 149-173.
- Ribeiro AM, Figueiras A, Freire C, Delfim S, Veiga F, et al. (2010) Combining strategies to optimize a gel formulation containing miconazole: The influence of modified cyclodextrin on textural properties and drug release. Drug Development and Industrial Pharmacy 36(6): 705-714.
- Seo YG, Dong WK, Yeo WH, Ramasamy T, Yu-Kyoung O, et al. (2013) Docetaxel-loaded thermosensitive and bioadhesive nanomicelles as a rectal drug delivery system for enhanced chemotherapeutic effect. Pharmaceutical Research 30(7): 1860-1870.
- Deng P, Feixiang C, Zhang H, Chen Y, Zhou J, et al. (2021) Conductive, self-healing, adhesive, and antibacterial hydrogels based on lignin/cellulose for rapid MRSA-infected wound repairing. ACS Applied Materials & Interfaces 13(44): 52333-52345.
- Ribeiro A, Veiga F, Delfim S, Juan JTL, Angel C, et al. (2011) Receptor-based biomimetic NVP/DMA contact lenses for loading/eluting carbonic anhydrase inhibitors. Journal of Membrane Science 383(1-2): 60-69.
- Ribeiro AM, Neumann IA (2017) Advances in composite hydrogels for ocular drug delivery and biomedical engineering application. Functional Hydrogels in Drug Delivery, CRC Press, USA, pp. 303-326.
- Kulkarni V, Kishor SDB, Rathod SS (2012) Natural polymers-A comprehensive review. Int J Res Pharm Biomed Sci 3(4): 1597-1613.
- Stanescu V (2011) Starch/chitosan film forming hydrogel. Rev Roum Chim 56(8): 827-832.
- Jung, YS, Wooram P, Hyejin P, Lee DK, Kun N, et al. (2017) Thermo-sensitive injectable hydrogel based on the physical mixing of hyaluronic acid and Pluronic F-127 for sustained NSAID delivery. Carbohydrate Polymers 156: 403-408.
- Akash MSH, Rehman K (2015) Recent progress in biomedical applications of Pluronic (PF127): Pharmaceutical perspectives. Journal of Controlled Release 209: 120-138.
- Dumortier G, Grossiord JL, Florence A, Jean CC (2006) A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharmaceutical Research 23(12): 2709-2728.
- Lauretis AD, Anne EA, Antara P, Pedersen JS, Szymon MS, et al. (2025) Balancing sterilization and functional properties in Poloxamer 407 hydrogels: Comparing heat and radiation techniques. Regenerative Biomaterials 12: rbaf005.
- El-Bagory IM (2010) Effect of gamma irradiation on pluronic gels for ocular delivery of ciprofloxacin: In vitro Australian Journal of Basic and Applied Sciences 4(9): 4490-4498.
- Manubolu, K, Raveesha P (2024) Formulation and in-vitro evaluation of vildagliptin microspheres using pectin and xanthan gum as polymers. Research Square.
- Dureja H (2023) Natural polymeric materials based drug delivery systems in lung diseases. Springer, Singapore.
- Kruk K, Winnicka K (2022) Alginates combined with natural polymers as valuable drug delivery platforms. Marine drugs 21(1): 11.
- Leone G, Barbucci R (2009) Polysaccharide based hydrogels for biomedical applications. Hydrogels: Biological Properties and Applications. Springer, Italy, pp. 25-41.
- Dash M, Chiellini F, Ottenbrite RM, Chiellini E (2011) Chitosan-A versatile semi-synthetic polymer in biomedical applications. Progress in Polymer Science 36(8): 981-1014.
- Sriamornsak P (2003) Chemistry of pectin and its pharmaceutical uses: A review. Silpakorn University International Journal 3(1-2): 206-228.
- Lee KY, Mooney DJ (2012) Alginate: Properties and biomedical applications. Progress in Polymer Science 37(1): 106-126.
© 2025 Andreza Maria Ribeiro. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






