- Submissions

Full Text
Modern Approaches in Drug Designing
Antioxidants Activity of Selected Synthesized Compounds
Jagessar RC*
Faculty of Natural Sciences, Department of Chemistry, University of Guyana, South America
*Corresponding author:Jagessar RC, Faculty of Natural Sciences, Department of Chemistry, University of Guyana, South America
Submission: January 03, 2025;Published: January 27, 2025

ISSN: 2576-9170 Volume4 Issue3
Abstract
Antioxidants have fulfilled many roles. In the health realm, they are used to prevent oxidative stress which is a causative factor in the pathophysiology of numerous diseases such as diabetes, cancer and cardiovascular diseases such as atherosclerosis. They are also said to promote the healthy life and longevity. There uses in the food industry are well known. Antioxidants can be synthetic and natural. There is a growing need to use plant-based antioxidants, since this would lead to further decarbonization and produce a greener environment and are much safer to use compare to synthetic antioxidants. Nevertheless, research in the design and synthesis of synthetic antioxidants are also on the rise to find more potent ones compared to standard models such as ascorbic acid. In addition, pharmaceutical companies need to promote their business via synthesis of new and potent pharmaceuticals with fewer side effects. Thus, synthetic antioxidants continue to rise. Amongst the ones reported to date include: Butylated Hydroxyl Anisole (BHA), Tert Butyl Hydroxyquinone (TBHQ), Propyl Gallate (PG), Chalcones, Indazole derivatives, Triazole derivatives, Pyrimidine derivatives, Schiff base derivatives, methoxyphenols derivatives, fulvic acid etc. Amongst the methods used to determine radical scavenging activity include the FRAP method, DPPH method, FIC (Ferrous Ion Chelation) method, phosphomolybdenum method etc.
Keywords:Antioxidant; Synthetic; Natural; Chalcones; Indazole derivatives; Triazole derivatives; Pyrimidine derivatives
Introduction
Antioxidants are chemical compounds or mixture of compounds which when present in low concentrations are used to prevent the oxidation of lipids, sugars, proteins and DNA that can generate aldehydes, ketones, esters and other products that can be harmful to living systems. Antioxidants can be synthetic or natural. Synthetic antioxidants include Butylated Hydroxyl Anisole (BHA), Tert-Butyl Hydroquinone (TBHQ) and Propyl Gallate (PG), etc [1]. The most common oxidants in biological systems are free radicals. Free radicals are atoms, molecules or ion that possess an unpair electron, seeking electrons from other atoms, molecules or ions in order to stabilize themselves. An initial attack causes the odd electron to be paired. However, this can result in the generation of another free radical and promoting a chain reaction [2]. In the biological systems, free radicals are derived from oxygen, nitrogen and sulphur molecules. They occur as parts of groups of molecules called Reactive Oxygen Species (ROS), Reactive Nitrogen Species (RNS) and Reactive Sulphur Species (RSS). ROS includes free radicals such as superoxide anion (O2-.), per hydroxyl radical, hydroxyl radical, nitric oxide, hydrogen peroxide, singlet oxygen, hypochlorous acid and peroxynitrite [3].
Free radicals are formed via three (3) major pathways. These include:
a. Homolytic cleavage of covalent bond of a normal molecule, with each fragment, retaining
one of the paired electrons.
b. By loss of a single electron from normal molecule.
c. By addition of a single electron to an atom, molecule.
They are usually formed in vivo [4] via:
a) Normal aerobic respiration in which the mitochondria
consume O2, reduces it by sequential steps to produce O2, H2O2
and OH- as by product.
b) Bacteria or virus infected cells get destroyed by phagocytosis,
with an oxidative burst of Nitric Oxide (NO), O2, H2O2 and OCl.
c) Peroxisomes producing H2O2 as a product of fatty acid and
other lipid molecular degradation. Its further degraded by
catalase. Some of the peroxide may be released into other cell
compartments, leading to increasing oxidative stress and DNA
damage.
d) Animal Cytochrome P450 which are the primary defence
systems that protect against toxic chemicals may generate
oxidative by products that will damage DNA.
Antioxidants have many uses. These include their medicinal uses whereby antioxidants retard ageing, promote longevity and the prevention of other diseases such as cancer and cardiovascular diseases that result from oxidative stress. They are also used as preservatives for foods [5].
Mini Review
Figure 1:Chalcone derivatives.
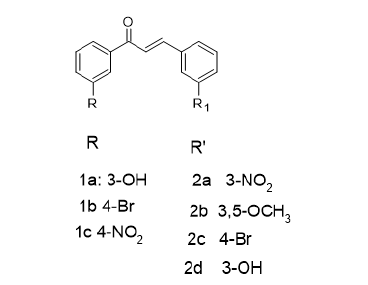
This paper is a mini review of the antioxidant activities of selected compounds. In one study, the synthesis, characterisation and evaluation of the antioxidant activities of some novel chalcones derivatives (1a-2d) as shown in Figure 1 have been reported [6]. In this report, a novel series of chalcones esters have been synthesized via the reaction of the requisite chalcones with either palmitic or stearic acid. Another class of chalcones comprising 2,3-disubstituted chalcones and 2-amino-6-(substituted-phenyl)- 4-substitutedphenyl-nicotinonitrile derivatives have also been prepared by electrophilic and Michael addition reactions with the respective chalcones. The structural integrity of compounds was established via 1HNMR, 13CNMR, IR and mass spectroscopy. The radical scavenging activities were investigated using 1,1-biphenyl- 2-picrylhydrazyl as the free radical scavenging agent. These compounds were selected on the basis that the above chalcones would quench the 1,1-biphenyl-2-picrylhydrazyl radical scavenging activities. Results indicated that one of the compounds (68.58% at C=2ug/ml) was more effective as an antioxidant agent than the reference antioxidant, ascorbic acid. The synthesis of new indazole derivatives, Figure 2, as potential antioxidants agents have been cited [7]. These derivatives were synthesized by reacting hydrazine hydrate and its derivatives with cyclohexanone derivatives. The latter were prepared from the respective chalcones via refluxing with ethyl acetoacetate in ethanol in the presence of sodium hydroxide. These compounds were characterized by 1HNMR, 13CNMR, IR and mass spectroscopy. All indazole derivatives exhibited radical scavenging activity against DPPH, reducing power capacity and total antioxidant capacity in comparison with respective standard (Figure 3).
Figure 2:Novel Indazole derivatives.
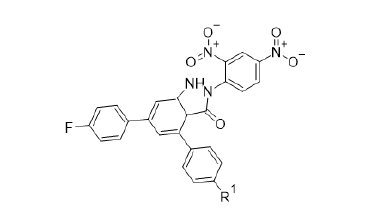
Figure 3:Novel Pyrimidine derivatives.
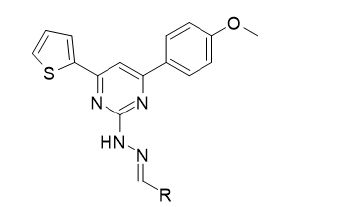
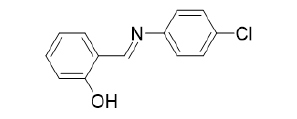
Novel derivatives of 4-R-5-phenethyl-4H-1,2,4-triazole- 3-thiols were synthesized. The antioxidant activity of the synthesized compounds was evaluated in vitro by the method of the non-enzymatic initiation of BOD with salts of iron (II). These compounds were found to be more potent than some of the existing antioxidants [8]. Synthesis and antioxidant activity of some Novel Pyrimidine Derivatives have been cited [9,10]. The percentage yield of the synthesized compounds ranges from 52-61%. All synthesized compounds were characterized via 1HNMR, 13CNMR and IR spectroscopy. Antioxidant activities were investigated via the DPPH scavenging method. 0.002% DPPH in methanol was used as the free radical. The absorbance was measured at 517nm using UV-Visible spectroscopy. The absorbance of the DPPH control was also noted. The scavenging activity of the compounds versus the stable DPPH was calculated using the equation: Scavenging activity (%)=(A-B)/AX100, where ‘A’ was the absorbance of DPPH solution and ‘B’ was the absorbance of DPPH solution with selected compounds. It was found that the Scavenging activity (%) of different concentrations (ug/mL), ranging from 25 to 400ug/mL, increased with increasing concentration of the compound, with 400ug/mL exhibiting the largest. At that concentration, the scavenging activity range from 64.23% to 96.11 %. The Schiff base ligand: (2-{(E)- [(4-chlorophenyl) amino] methyl} phenol), Figure 4 and its M(II) complexes (M=Cu, Ni, Zn and Co) have been synthesized and their antioxidant properties evaluated via the FRAP, DPPH, FIC (Ferrous Ion Chelation) and phosphomolybdenum method by hydroxyl free radical (•OH) scavenging activity.
Figure 4:(2-{(E)-[(4-chlorophenyl) imino] methyl} phenol).
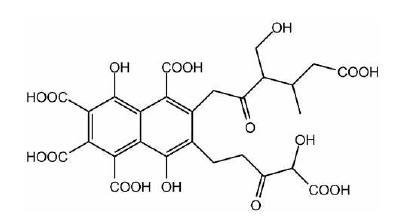
The phosphomolybdenum assay involves the reduction of Mo (VI) to Mo(V) by the selected antioxidant compound, resulting in the formation of a green precipitate-Mo(V) complex in acidic solution. It was observed that the synthesized compounds show significant antioxidant properties as compared to conventional antioxidants such as Vitamin-C and EDTA [11]. Fulvic Acid (FA) is a class of compound including humic substances together with humic acid and humin. The structure of Fulvic acid is shown in Figure 5. It is formed via the degradation of organic substances by chemical and biological process. FA consists of a mixture of closely related complex aromatic polymers with the presence of aromatic rings, phenolic hydroxyl, ketone carbonyl, quinone carbonyl, carboxyl and alkoxyl groups. For the first time, the scavenging activity of biosynthesized fulvic acid in comparison with reference compounds has been noted. FA displays a scavenging activity compared with the reference compounds although it was less efficient than Nordihydroguaiaretic Acid (NDGA), ascorbic acid, pyruvate, Di Methyl Thio Urea (DMTU), penicillamine and Glutathione (GSH) for O2. Thus, FA is a good candidate to be used in pharmaceutical or food industries as an accessible synthetic and natural antioxidants [12]. Oxidative stress is the source for numerous diseases, such as diabetes, atherosclerosis, cancer, neurodegenerative and cardiovascular diseases. Therapeutic antioxidants are promising candidates for preventing and treating oxidative stress and thus prevent the above diseases. In response to this, the design, synthesis and antioxidant activity of six compounds containing the 2-methoxyphenol moiety has been reported [13]. These compounds were synthesized in yields of 48-95%. The synthesized derivatives were characterized using 1H NMR, 13C NMR, Fourier- Transform Infrared (FT-IR) spectroscopy and elemental analyses. The antioxidant properties of the compounds were evaluated using the 2,2-Diphenyl-1-Picryl-Hydrazyl-Hydrate (DPPH), 2,2’-Azino- Bis (3-Ethylbenzothiazoline-6-Sulfonic Acid (ABTS), and Oxygen Radical Absorbance Capacity (ORAC) assay. All six (6) compounds showed strong antioxidant activity using the three assays above.
Figure 5:The structure of fulvic acid.
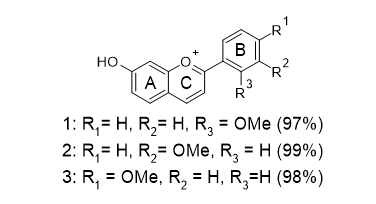
Anthocyanidins are coloured dyes that are responsible for the bright colour of fruits and flowers. They belong to the flavonoid family and the structure comprise of three rings: A, B and C. Aromatic ring (A) is fused with heterocyclic ring, containing and oxygen (C), which is bonded to a third aromatic ring (B). In one report, Anthocyanidins (1) to (3) were synthesized in excellent yield (97-98%) to study the effect of methoxy substitution on the B ring with respect to their antioxidant properties (Figure 6). Comparative FRAP studies show 2′- and 4′-methoxy substituents have higher antioxidant activities, which may be attributed to both resonance and inductive effects [14]. In recent years, developing phenolic antioxidants that are potent is a very active area of research. However, the use of phenolic compounds has also been limited by poor antioxidant activity in several in vivo research. Polymeric phenols have received much attention owing to their potent antioxidant properties and increased stability in aqueous systems. One approach to synthesise polyphenolic antioxidants via enzyme catalysed synthesis of polymeric phenols [15].
Figure 6:
Conclusion
The design and synthesis of compounds with antioxidant activities has been increasing with the view to find potent antioxidants with least side effects. Synthetic antioxidants are not environmentally safe, coupled with their arduous synthesis and should be superseded with herbal antioxidants. Some of the synthesised antioxidants reported to date are: Butylated Hydroxyl Anisole (BHA), Tert Butyl Hydroxyquinone (TBHQ), Propyl Gallate (PG), Chalcones, indazole derivatives, triazole derivatives, pyrimidine derivatives, Schiff base derivatives, fulvic acid etc. They have shown variation in antioxidant potency compared to reference standard such as ascorbic acid. In some cases, lesser and in others greater. Amongst the methods used to determine radical scavenging activity include the FRAP, DPPH, FIC (Ferrous Ion Chelation) and phosphomolybdenum method.
References
- Jayathilakan K, Sharma GK, Radhakrishna K, Bawa AS (2007) Antioxidant potential of synthetic and natural antioxidants and its effect on warmed-over-flavour in different species of meat. Food Chem 105(3): 908-916.
- Matill HA (1947) Antioxidants. Ann Rev Biochem 16: 177-192.
- Vajragupta O, Boonchoong P, Berliner LJ (2004) Manganese complexes of curcumin analogues evaluation of hydroxyl radical scavenging ability superoxide dismutase activity and stability towards hydrolysis. Free Read Res 38(3): 303-314.
- Hudson BJF (1990) Food antioxidants. (1st edn), Elsevier Science Publishing CO, Amsterdam, Netherlands.
- Emad M Atta, NH Mohamed, Ahmed AM Abdelgawad (2017) Antioxidants: An overview on the natural and synthetic types. Eur Chem Bull 6(8): 365-375.
- Lahsasni SA, Al Korbi FH, Alijaber NA (2014) Synthesis characterisation and evaluation of antioxidant activities of some novel chalcones analogues. Chemistry Central Journal 8: 32.
- Sapnakumari M, Narayana B, Sarojini BK, Madhu LN (2014) Synthesis of new indazole derivatives as potential antioxidant agents. Med Chem Res 23: 2368-2376.
- Ihnatova T, Kaplaushenko A, Frolova Y, Pryhlo E (2021) Synthesis and antioxidant properties of some new 5-phenethyl-3-thio-1,2,4-triazoles. Pharmacia 68(1): 129-133.
- Siddesh MB, Padmashali B, Thriveni KS, Sandeep C, Goudarshivnnanavar BC (2013) Synthesis and pharmacological evaluation of some novel pyrimidine derivatives. Journal of Applicable Chemistry 2(5): 1281-1288.
- Siddesh MB, Padmashali, B, Thriveni KS, Sandeep C, Nagesh HK, et al. (2014) Synthesis of polynuclear pyrimidine derivatives and their pharmacological activities. Universal Journal of Pharmacy 4(4): 503-514.
- Ibrahim M, Khan A, Ikram M, Rehman S, Shah M, et al. (2017) In vitro antioxidant properties of novel Schiff base complexes. Asian J Chem Sci 2(2): 1-12.
- Rodriguez NC, Urrutia EC, Gertrudis BH, Chaverri JP, Mejia GB (2011) Antioxidant activity of fulvic acid: A living matter-derived bioactive compound. Journal of food agriculture & environment 9(3): 123-127.
- AlNeyadi SS, Amer N, Thomas TG, Ajeil RA, Breitener P (2020) Synthesis characterization and antioxidant activity of some 2-methoxyphenols derivatives. Heterocyclic Communications 26(1): 112-122.
- Barcena HS, Chen P, Tuachi A (2015) Synthetic anthocyanidins and their antioxidant properties. Springer Plus 4: 499.
- Nagarajan S, Nagarajan R, Kumar J, Salemme A, Togna AR, et al. (2020) Antioxidant activity of synthetic polymers of phenolic compounds. Polymers 12(8): 1646.
© 2025 Jagessar RC. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






