- Submissions

Full Text
Journal of Biotechnology & Bioresearch
Chitosan- Based Materials with Antibacterial Activities As a Biotechnological Material for Biomedical Applications: A Mini- Review
Mohammad Reza Kasaai*
Department of Food Science and Technology, Sari Agricultural Sciences and Natural Resources University, Iran
*Corresponding author:Mohammad Reza Kasaai, Department of Food Science and Technology, Sari Agricultural Sciences and Natural Resources University, Sari, Km. 9, Farah Road, 48181- 68984, Sari, Mazandaran Province, Iran
Submission: June 16, 2025;Published: September 15, 2025

Volume5 Issue 5August 05, 2025
Abstract
Use of biopolymers, as alternatives to synthetic polymers, offers significant advantages on both living and environmental systems, by reducing and minimizing potential risks. Biopolymers represent safe materials that can replace synthetic ones in several branches of medicine. Among natural polymers, chitosan is unique, due to its biocompatibility, safety and Antibacterial Activity (ABA). Chitosan serves as a safe alternative inhibitor that can substitute synthetic antibiotics, which are associated with various side effects and the development of resistance. This mini-review focuses on the ABA of chitosan as an inhibitor against various bacteria, those are harmful for living systems particularly for humans. Properties, function and biomedical and health care applications of bulk and nanosized of chitosan were presented. The effects of chitosan on gram-positive- and gram-negative bacteria in vitro, and in vivo have been discussed. The inhibitory effects of chitosan with some antibiotics were compared. The effects of molecular weight (Mw), Degree of N-Acetylation (DA) of chitosan, on ABAs have been also discussed. Use of a variety of chitosan derivatives, composite of chitosan, and chitosan- based materials possessing ABAs in different branches of medicine have been described. The topic not only for different branches of medicine but also for other fields of biotechnology (agriculture, and environment) is of interest
Keywords:Chitosan; Antibacterial; Medical applications
Introduction
The long-term use of antibiotics against micro-organisms results in the development of microorganism resistance [1-4]. Consequently, it is essential to investigate innovative strategies for the development of effective and safe therapies to treat bacterial infections and to substitute harmful antibiotics. Natural Antibacterial Compounds (ABCs) are safe alternatives to antibiotics for treatments of various diseases arising from infections. Due to biocompatibility and non-toxicity of biopolymers, they can be used in medical applications [5,6]. They are widely used in pharmaceutical industries for the formulation of controlled release systems, as well as in artificial muscles and wound healings, due to their non-toxicity, biodegradability and biocompatibility [7]. Among biopolymers, chitosan stands out a unique, due to its cationic nature (amino groups), and Antimicrobial Activity (AMA). The Antibacterial Activity (ABA), make chitosan highly versatile, with applications across various fields of medicine such as various bacterial infections, wound dressing, tissue engineering, and drug delivery systems [8]. Nano-Sized Materials (NMs) exhibit physicochemical properties significantly different from those of their original counterparts [9,10]. Additionally, NMs create greater efficiencies at shorter periods in target therapies compared to the bulk counterparts [11]. For instance, nanosized of chitosan and its Nanocomposites (NCs) with carboxymethyl cellulose inhibit the growth of a variety of bacteria with a superior ABA and a greater efficiency compared to the original chitosan [12]. This manuscript provides a minireview of several reports mainly published between 2010-2025 on chitosan as an ABC to protect humans from bacterial infections. Several subjects have been described as follows: (1) chitosan as a safe alternative to synthetic antibiotics; (2) an analysis of the differences between chitosan and chitosan Nanoparticles (NPs)/ NMs, focusing on how the size of chitosan influences its efficiency in ABAs; (3) conduct a comparison of the ABA of chitosan and its derivatives; (4) conduct a comparison of the ABA of chitosan and its combinations with other active compounds; and (5) use of chitosan, chitosan derivatives, and chitosan- based materials for treatment of various bacterial infections.
General Aspects
Preparation of chitosan and its chemical structure
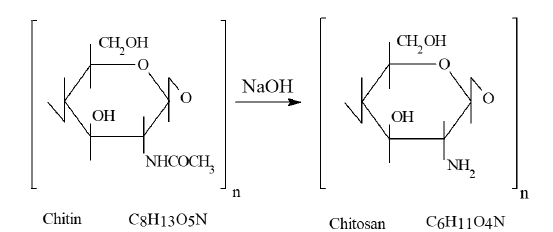
Chitin is the second most abundant polysaccharide in nature, after cellulose, often is regarded as a cellulose derivative. It is insoluble in most common organic and inorganic solvents. The conversion of chitin into chitosan significantly enhances its solubility and facilitates its processing, thereby expanding its range of practical applications. Chitosan is produced from chitin through the N-deacetylation as illustrated in Figure 1. The term chitosan is used for both partially and fully deacetylated forms. Usually, chitosan is a partially N-deacetylated chitin and comprises two subunits[glucosamine, and N-acetyl glucosamine, linked via β (1–4)-glycosidic linkages]. These two biopolymer units randomly distributed along macromolecule chains [13-19].
Figure 1:Preparation of chitosan by N-deacetylation of chitin.
Properties of chitosan
Table 1:Resources, characteristics, properties, functions and medical and health care applications of bulk and nanosized of chitosan.

Resources, characteristics, properties, functions and medical and health care applications of bulk and nanosized of chitosan are presented in Table 1. Chitosan with positively charged amino groups (NH3+) exhibits AMA, including Antibacterial Activity (ABA) against both gram-negative and gram-positive bacteria and Antifungal Activity (AFA) [20-22]. The amino groups of chitosan can interact with negatively charged of cell walls of micro-organisms, followed by penetrate to the cells. and ultimately prevent the growth or death of the cells . Additionally, chitosan exhibits film and fiber forming properties, making it suitable for constructing hydrogels, nanofibers, and membranes [21,23-25]. Two critical structural parameters influencing the biological properties of chitosan are the degree of N-acetylation (DA) and molecular weight (Mw). Generally, chitosan with a lower DA exhibits superior ABA compared to chitosan with a higher DA. Chitosan with a low DA or a low Mw in a diluted organic acid is converted into positive charged as a result of the protonation of the amino groups. Additionally, the distribution of two units, D-glucosamine; and N-acetyl-Dglucosamine, along macromolecular chains produce various types of sequences: random; block; or alternated block macromolecules having different charged densities as well as different level of ABAs. The solubility of chitosan not only depends on the DA and Mw, but also depends on the distribution of the monomer units, and pH of medium. The quaternary salts of chitosan demonstrate improved solubility in both acidic and alkaline solutions and enhanced Antibacterial Properties (ABPs) [15,26]. The ABA of chitosan and its derivatives is influenced by physicochemical properties and external environmental conditions such as solubility of chitosan and pH of chitosan solutions [1,27].
Use of Nanoscience and Nanotechnology in Medicine
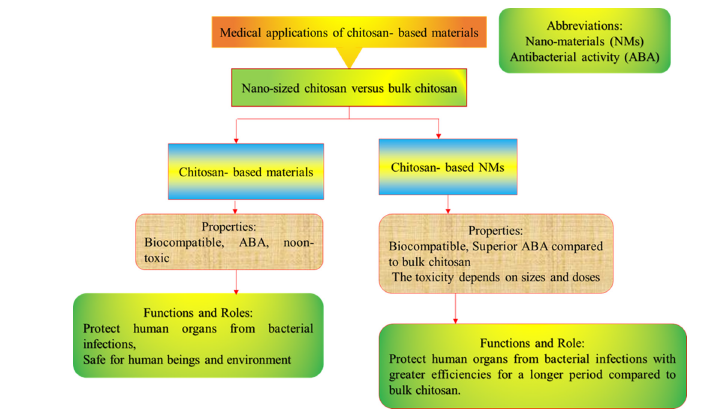
Nanotechnology is widely regarded as one of the key technologies of the 21st century, offering significant potential in medicine. It plays a critical role in the development of advanced medicine for several treatments of diseases. The ABAs of chitosan and its derivatives significantly enhance by using nanosized chitosan [1,25,28-30]. Nanosized of chitosan and its NCs with carboxymethyl cellulose inhibit a variety of bacteria with a superior ABAs and a greater efficiency compared to the original chitosan [12]. Silver NPs (Ag NPs) exhibit Antimicrobial Properties (AMPs) against various microorganisms and can combine with chitosan. Results showed Ag NPs enhanced ABPs against S. aureus and E. coli. The ABPs increased significantly as the Ag NPs content increased [31,32]. Oligomeric chitosan having nanosized can easily penetrate the cell membrane of a microorganism and once inside, inhibits RNA transcription and prevents the growth of the cell [24]. A comparison of some properties particularly ABAs of nanosized chitosan versus bulk chitosan was illustrated in Figure 2.
Figure 2:A comparison of some properties particularly ABAs of nanosized chitosan versus bulk chitosan.
Nanosized of chitosan
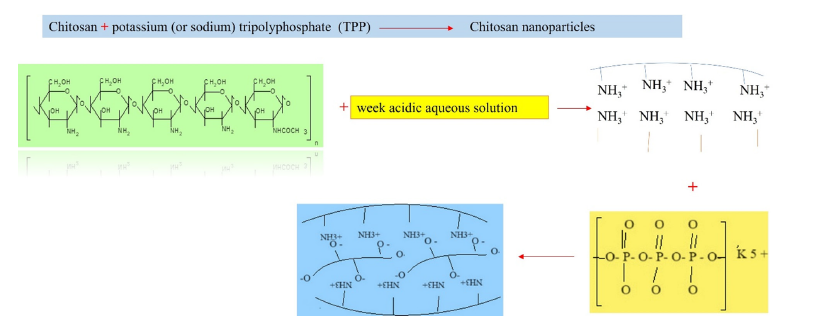
Chitosan NPs have been usually fabricated by a gelation procedure via electrostatic interactions between positively charged of amino groups (-NH3+) of chitosan and negatively charged of sodium Tripolyphosphate (TPP) [33]. The TPP is non-toxic and thus the fabrication of chitosan NPs is a safe procedure. Chitosan NPs is much safer, when it is made with food-grade of TPP [11]. Both chitosan: TPP ratio and pH of chitosan solution are affected the physicochemical characteristics [size, Polydispersity Index (PDI), Zeta Potential (ZP), surface charge)] of the NPs. Chitosan NPs or chitosan Nanomaterials (NMs) create longer paths and stronger structures, and resulting nano- reinforced materials with enhanced MPs in comparison with conventional ones [34,35]. TPP-crosslinked chitosan NPs formed a more compact, denser coating layer, and smaller hydrodynamic volume than that of the original chitosan [36]. Figure 3 shows fabrication of chitosan NPs from chitosan and TPP. Chitosan NPs demonstrate a considerable compatibility with metal ions (Ag, Zn, Cu), and their combinations, yield in NCs with enhanced ABAs, and expand their applications [30-32,37].
Figure 3:Fabrication of chitosan- nanoparticles from chitosan and sodium or potassium tripolyphosphate (TPP).
Antibacterial Activity of Chitosan- Based Materials
Several bacteria cause human infections
The most common pathogenic bacteria involve human infections include gram-positive [Enterococcus faecalis (E. faecalis), Staphylococcus aureus (S. aureus),- MSSA ATCC 29213, and S. aureus- MRSA ATCC 43300), and gram-negative [Escherichia coli (E. coli), Klebsiella pneumoniae (K.pneumoniae), Pseudomonas aeruginosa (P. aeruginosa)] bacteria. The susceptibility of these bacteria to Antibacterial Compounds (AMCs) is different, due to their specific cell wall structures and morphologies [38].
Chitosan-based materials as antibacterial agents for treatment of various infections
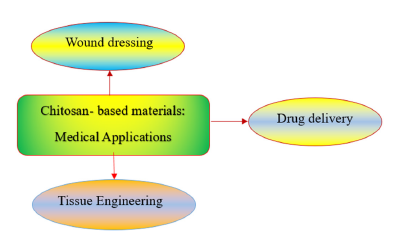
Applications of chitosan- based materials with ABAs in three major branches of medicine are illustrated in Figure 4. Chitosan derivatives possessing Quaternary Ammonium Groups (QAGs) demonstrated superior ABAs compared to the neat chitosan. Chitosan derivatives with entirely ammonium ions make them very effective in inhibition of various bacteria. The main benefit of chitosan derivatives possessing QAGs over other chitosan derivatives is its fixed cationic charged groups. The strong electrostatic interactions between the QAGs of the chitosan derivatives and functional groups of bacterial cell membranes, leading membrane cleavage and thus destroy the integrity of the cell membrane. E. coli, a gram-negative, and S. aureus, a gram-positive, bacteria have been selected as model microorganisms to evaluate the ABA of chitosan derivatives. Minimal Inhibitory Concentration (MIC) and Minimal Bactericidal Concentration (MBC) have been determined to evaluate the ABAs of chitosan, and its derivatives. The inhibitory effect of chitosan derivatives containing QAGs on S. aureus was found to be more pronounced than that on E. col. In a mildly alkaline environment, the proliferation of E. coli was less inhibited compared to S. aureus [1,27]. Variations in the components of the cell walls influence the mechanism of action of chitosan and its derivatives. Peptidoglycan (PG) serves as the primary structural element of the cell wall in S. aureus, made up of alternating residues of N-Acetylmuramic acid (MurNAc) and N-Acetylglucosamine (GlcNAc). The cellular architecture of E. coli is characterized by two distinct membranes: the outer membrane and the inner membrane. The outer membrane consists of proteins, phospholipids, and Lipopolysaccharides (LPS). The QAGs of chitosan derivatives interact with the negatively charged phospholipid components of the bacterial cytoplasmic membrane, leading to significant disruption of the lipid bilayer, which can result in cytoplasmic leakage, lysis, and ultimately cell death. Chitosan derivatives having QAGs have potential applications in drug delivery and wound dressing [1,27].
Figure 4:Applications of chitosan- based materials with ABAs in three branches of medicine.
Two reactive functional groups (amino groups on the C2 of deacetylated units, and hydroxyl groups on the C3 and C6 of both acetylated and deacetylated units) of chitosan, create several possibilities for structural modifications and preparation of several derivatives, hydrogels micro/NPs, and composite/ NCs, for a variety of applications [21-22,39-43]. Hydrogels can be prepared through the interactions between functional groups of chitosan and functional groups of cross-linking agents. The specific type of cross-linking agent determines whether amino, hydroxyl, or both groups of chitosan are involved in the cross-linking process. For instance, the NPs of hydrogels were developed using a cross-linking agent, TPP. The hydrogels were characterized by Fourier-Transform Infrared Spectroscopy (FTIR), Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), Thermal Gravimetry Analysis (TGA) and differential scanning calorimetry (DSC). The hydrogels were tested versus eight bacteria, including gram- positive [Bacillus subtilis (B. subtilis, ATCC 6633), S. aureus, ATCC 6538, Staphylococcus epidermidis (S. epidermidis, ATCC 12228) and Streptococcus pyogenes (S. pyogenes, ATCC 19615)], and gram-negative [E. coli, ATCC 11229, K. pneumoniae, ATCC 10145, Proteus (ATCC 33420), and Salmonella enteritidis (S. enteritidis, ATCC 7251)] bacteria using nutrient agar method [44]. The experimental results indicated that both chitosan hydrogels and chitosan hydrogel NPs exhibited greater ABAs than chitosan and chitosan NPs alone. Notably, chitosan hydrogel NPs demonstrated the highest MIC and MBC, particularly against S. pyogenes, with values of 19.5 and 39 μg.ml-1, respectively, in comparison to the standard antibiotic Ciprofloxacin, which had MIC and MBC values of 19 and 38 μg.ml-1. Ciprofloxacin is a fluoroquinolone antibiotic used to treat a number of bacterial infections. Chitosan-gelatin composite exhibited enhanced ABA compared to the neat chitosan. Formation of strong hydrogen bonds between chitosan and gelatin, leading a closed and dense structure with enhanced ABA [45]. The release of bioactive substances from the chitosan basted matrix is influenced by different physicochemical properties, including the pH of solution or the protonation of amine groups of chitosan macromolecule chains [8, 46,47].
Wound healing and tissue engineering
Infection of wounds can delay the healing process. Chitosan having broad-spectrum of ABAs, helps reduce this risk [25,48]. A combination of chitosan and Cu²⁺exhibits a synergistic antibacterial effect. The addition of Cu²⁺to chitosan- based hydrogels strengthens their adhesiveness, while also improving their ABAs [37]. Additionally, a combination of chitosan oligosaccharide and oxidized hyaluronic acid produced nanogels, and facilitated the release of antibiotics such as enrofloxacin for the treatment of infected wounds [49]. A chitosan- based NCs membrane demonstrated superior ABA and has been used for wound healing infections. A membrane comprises of chitosan and zeolitic imidazolate framework-8 (ZIF-8) was designed. ZIF- 8 is a metal-organic framework constructed from zinc ions and imidazolate linkers. The membrane exhibited superior ABA against S. aureus and has been used for wound-infected rat models. ZIF-8 is characterized by its high porosity and biocompatibility. The pHsensitive decomposition of ZIF-8 NPs enables targeted drug release in acidic medium, such as those commonly present in infected wounds. ZIF-8 not only enables regulated drug delivery under acidic conditions but also demonstrates ABPs by slowly releasing zinc ions. The dual functionality of ZIF-8 in combination with chitosan makes this NCs a valuable healing compound for wound care applications [25,50]. A hydrogel made from chitosan derivative demonstrated ABA with a reduction in antibiotic resistance. This hydrogel was developed with a combination of ZIF-8, polydoamine - carbolxylated chitosan. The hydrogel exhibited photocatalytic and photothermal antibacterial effects; The hydrogel totally inhibited the bacterial growth via elevated local temperatures. The temperature was raised over 70°C, when exposed to the nearinfrared light, while the regulated release of Zn2+ ions facilitated healing, and diminished antibiotic resistance. This hydrogel has been used for wound healing infections [51].
Conclusion
This mini-review focuses on ABA of chitosan, its derivatives and chitosan -based materials, both bulk and nanosized for applications in various branches of medicine. Biocompatibility, biodegradability, ABA, and ability to form functional nanostructures makes chitosan a highly versatile biopolymer. Chitosan, its derivatives, its composites and chitosan- based materials showed significant potential for a wide range of applications across various branches of medicine and healthcare such as wound dressing, tissue engineering and drug delivery. Chitosan demonstrated an inhibitory effect on the growth of various bacteria, including both gram-positive and gramnegative bacteria. The level of inhibition increased as the DA of chitosan decreased. Among chitosan samples with similar Mw, but differing DAs, those with a DA of 0.0 or those containing entirely amino groups exhibited the highest ABA. Additionally, among all forms of chitosan, those containing entirely ammonium ion forms or the quaternary salts of chitosan displayed the most significant ABAs. Nanosized chitosan or chitosan NPs can penetrate and be absorbed by human organs more efficiently and rapidly than bulk chitosan, resulting in improved efficacy and performance. Moreover, the nanoscale dimensions of chitosan provide a high surface area-to-volume ratio, facilitating interactions with targeted human organs, whereas bulk chitosan encounters challenges in interacting with human organs, often resulting in minimal both interactions and efficiencies. Chitosan-based NMs with superior properties compared to bulk materials, open innovative strategies in several branches of biotechnology, medicine and health care sectors, such as diagnostics, therapeutics, drug deliveries, wound healing and tissue engineering. Additionally, they protect food and agricultural products from several pathogens.
Future Perspectives
There is a little information on the safety of biopolymers such as chitosan at the nanoscale. A gap between safety and existing toxicity, direct us to design experimental strategy.In vitro nanotoxicity investigations are necessary to predict possible side effects, and to understand how NMs interact with biological systems. Clinical trials on the toxicity of chitosan NMs and chitosan- based NMs should be carried out in vivo before they are commercialized. Eliminating the risks related to exposure to the NMs is imperative. By confirming their safety, these materials become extremely effective and numerous applications in nanomedicine and healthcare become possible (Table 2).
Table 2:

Author Contributions
A sole author was prepared this review manuscript.
Conflicts of Interest
The author declares that there is no conflicts of interests regarding the publication of this manuscript.
Funding
This study did not receive any specific grants from funding agencies in the public, commercial, or not-for profit sectors.
References
- Mirbagheri VS, Alishahi A, Ahmadian G, Petroudi SHH, Ojagh SM, et al. (2023) Recent findings in molecular reactions of E. coli as exposed to alkylated, nano- and ordinary chitosans. Int J Biol Macromol 253: 127006.
- Raven PH, Johnson GB (2002) Biology, (6th edn), McGraw Hill, Boston, USA.
- Rosato A, Barbarossa A, Mustafa AM, Bonacucina G, Perinelli DR, et al. (2021) Comprehensive evaluation of the Antibacterial and antifungal activities of Carlina acaulis L. essential oil and its nanoemulsion. Antibiotics 10(12): 1451.
- Van den Bogaard AE, Stobberingh EE (2000) Epidemiology of resistance to antibiotics: Links between animals and humans. Int J Antimicrob Agents 14(4): 327-335.
- Behrooznia Z, Nourmohammadi J (2024) Polysaccharide-based materials as an eco-friendly alternative in biomedical, Environmental, and Food Packaging. Giant 19: 100301.
- Roberts EL, Abdollahi S, Oustadi F, Stephens ED, Badv M (2023) Bacterial-nanocellulose-based biointerfaces and biomimetic constructs for blood-contacting medical applications. ACS Mater Au 3(5): 418-441.
- Kasaai MR (2020) Biopolymer-based nanomaterials for food, nutrition, and healthcare sectors: An overview on their properties, functions, and applications, In: Hussain CM (Ed), Handbook of Functionalized Nanomaterials for Industrial Applications: Micro and Nano Technologies, pp. 167-184.
- Pérez-Álvarez L, Ruiz-Rubio L, Vilas-Vilela JL (2020) Chitosan-based nanogels for biomedical applications. In: Torchilin V (Ed), Handbook of Materials for Nanomedicine: Polymeric Nanomaterials, Jenny Stanford Publishing Pte Ltd, Singapore 8: 355-416.
- Kasaai MR (2018) Zein and zein -based nano-materials for food and nutrition applications: A review. Trends Food Sci Technol 79: 184-197.
- Mahkam M (2010) Starch-based polymeric carriers for oral-insulin delivery. J Biomed Mater Res 92(4): 1392-1397.
- Kumar N, Kumbhat S (2016) Essentials in nano-science & nanotechnology. New Jersey, John Wiley & Sons, USA.
- Jannatyha N, Shojaee-Aliabadi S, Moslehishad M, Moradi E (2020) Comparing mechanical, barrier and antimicrobial properties of nanocellulose/CMC and nanochitosan/CMC composite films. Int J Biol Macromol 164: 2323-2328.
- Jimenez-Gomez CP, Cecilia JA (2020) Chitosan: A natural biopolymer with a wide and varied range of applications. Molecules 25(17): 3981.
- Kasaai MR (2008) A review of several reported procedures to determine the degree of n-acetylation for chitin and Chitosan using infrared spectroscopy. Carbohydr Polym 71(4): 497-508.
- Kasaai MR (2009) Various methods for determination of the degree of n-acetylation of chitin and chitosan: A review. J Agr Food Chem 57(5): 1667-1676
- Marsili L, Dal Bo M, Berti F, Toffoli G (2021) Chitosan-based biocompatible copolymers for thermoresponsive drug delivery systems: On the development of a standardization system. Pharmaceutics 13(11): 1876.
- Palani N, Vijayakumar P, Monisha P, Ayyadurai S, Rajadesingu S (2024) Electro-spun nanofibers synthesized from polymers incorporated with bioactive compounds for wound healing. J Nanobiotechnology 22(1): 211.
- Bento R, Pagán E, Berdejo D, de Carvalho RJ, García-Embid S, et al. (2020) Chitosan nano-emulsions of cold-pressed orange essential oil to preserve fruit juices. Int J Food Microb 331: 108786.
- Divya K, Vijayan S, George TK, Jisha MS (2017) Antimicrobial properties of chitosan nanoparticles: Mode of action and factors affecting activity. Fibers and Polymers 18: 221-230.
- Jiang A, Patel R, Padhan B, Palimkar S, Galgali P, et al. (2023) Chitosan based biodegradable composite for antibacterial food packaging application, Polymers 15(10): 2235.
- Kasaai MR (2022) Bio-nano-composites containing at least two components, chitosan and zein, for food packaging applications: A review of the nano-composites in comparison with the conventional counterparts. Carbohydr Polym 280: 119027.
- Kumar S, Mukherjee A, Dutta J (2020) Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives, Trends Food Sci Tech 97:196-209.
- Carpa R, Farkas A, Dobrota C, Butiuc-Keul A (2023) Double-network chitosan- based hydrogels with improved mechanical, conductive, antimicrobial, and antibiofouling properties. Gels 9(4): 278.
- Klaykruayat B, Siralertmukul K, Srikulkit K (2010) Chemical modification of chitosan with cationic hyperbranched dendritic polyamidoamine and its antimicrobial activity on cotton fabric. Carbohydr Polym 80(1): 197-207.
- Tarin M, Akbari Oryani M, Javid H, Hashemzadeh A, Karimi-Shahri M (2025) Advancements in chitosan-based nanocomposites with ZIF-8 nanoparticles: Multifunctional platforms for wound healing applications. Carbohydr Polym 362: 123656.
- Biswas UK, Bose A, Ghosh B, Sharma S (2025) An insight into chemically modified chitosan and their biological, pharmaceutical, and medical applications: A review. Int J Biol Macromol 303: 140612.
- Cui J, Sun Y, Wang L, Ji Y, Zhao H, et al. (2025) Quaternary ammonium salts of chitosan containing aromatic ring: Synthesis, characterization, antimicrobial, antioxidant and cytotoxicity. Carbohydr Res 552: 109431.
- Cho H, Lai TC, Kwon GS (2013) Poly (ethylene glycol)-block-poly (ε-caprolactone) micelles for combination drug delivery: evaluation of paclitaxel, cyclopamine and gossypol in intra-peritoneal xeno-graft models of ovarian cancer. J Control Release 166(1): 1-9.
- Cho H, Lai TC, Tomoda K, Kwon GS (2015) Polymeric micelles for multi-drug delivery in cancer. AAPS Pharm Sci Tech 16 (1): 10-20.
- Mody VV, Siwale R, Singh A, Hardik R, Mody HR (2010) Introduction to metallic NPs. J Pharm Bioallied Sci 2(4): 282-289.
- An J, Zhang M, Wang S, Tang J (2008) Physical, chemical, and microbiological changes in stored green asparagus spears as affected by coating of silver nanoparticles-PVP. LWT 41(6): 1100-1107.
- Imran M, Ehrhardt CJ, Bertino MF, Shah MR, Yadavalli VK (2020) Chitosan stabilized silver nanoparticles for the electrochemical detection of lipopolysaccharide: A facile biosensing approach for gram-negative bacteria. Micromachines 11(4): 413.
- de Pinho Neves AL, Milioli CC, Müller L, Riella HG, Kuhnen NC, et al. (2014) Factorial design as tool in chitosan nanoparticles development by ionic gelation technique. Colloids Surf A: Physicochem Eng Asp 445: 34-39.
- Mihindukulasuriy SDF, Lim LT (2014) Nanotechnology development in food packaging: A review. Trends Food Sci Tech 40(2): 149-167.
- Souza VGL, Fernando AL (2016) Nanoparticles in food packaging: Biodegradability and potential migration to food-A review. Food Packag Shelf Life 8: 63-70.
- Li J, Huang Q (2012) Rheological properties of chitosan tripolyphosphate complexes: From suspensions to microgels. Carbohydr. Polym 87(2): 1670-1677.
- Wang L, Wang H, Dang H, Niu B, Yan H, et al. (2025) An adhesive, antibacterial hydrogel wound dressing fabricated by dopamine-grafted oxidized sodium alginate and methacrylated carboxymethyl chitosan incorporated with Cu(II) complex. Biomater Adv 170: 214217.
- Man A, Santacroce L, Iacob R, Mare A, Man L (2019) Antimicrobial activity of six essential oils against a group of human pathogens: A comparative study. Pathogens 8(1): 15.
- Liu Z, Wang S, Liang H, Zhou J, Zong M, et al. (2024) A review of advancements in chitosan-essential oil composite films: Better and sustainable food preservation with biodegradable packaging. Int J Biol Macromol 274(2): 133242.
- Ma Z, Garrido-Maestua A, Jeong KC (2017) Application, mode of action, and in vivo activity of chitosan and its micro and nanoparticles as antimicrobial agents: A review. Carbohydr Polym 176: 257-265.
- Priyadarshi R, Sauraj V, Kumar B, Deeba F, Kulshreshtha A, et al. (2018) Chitosan films incorporated with Apricot (Prunus armeniaca) kernel essential oil as active food packaging material, Food Hydrocoll 85: 158-166.
- Rahman PM, Abdul Mujeeb VM, Muraleedharan K, Thomas SK (2018) Chitosan/nano ZnO composite films: Enhanced mechanical, antimicrobial and dielectric properties. Ara J Chem 11(1): 120-127.
- Tan YM, Lim SH, Tay BY, Lee MW, Thian ES (2015) Functional chitosan-based grapefruit seed extract composite films for applications in food packaging technology. Mater Res Bull 69: 142-146.
- Ahmed ME, Mohamed HM, Mohamed MI, Kandile NG (2020) Sustainable antimicrobial modified chitosan and its nanoparticles hydrogels: Synthesis and characterization, International Journal of Biological Macromolecules 162: 1388-1397.
- Benbettaieb N, Kurek M, Bornaz S, Debeaufort F (2014) Barrier, structural and mechanical properties of bovine gelatin-chitosan blend films related to biopolymer interactions. J Sci Food Agri 94(12): 2409-2419.
- Castro-Munoz R, Kharazmi MS, Jafari SM (2023) Chitosan-based electrospun nanofibers for encapsulating food bioactive ingredients: A review. Int J Biol Macromol 245: 125424.
- Peers S, Montembault A, Ladaviere C (2020) Chitosan hydrogels for sustained drug delivery. J Control Release 326: 150-163.
- Kasaai MR (2019) Chitosan-based materials for wound healing and tissue engineering: An overview on their properties and applications, J Biotech Biores 2(1): JBB.000526.
- Luo W, Jiang Y, Liu J, Ju M, Algharib SA, et al. (2023) On-demand release of enrofloxacin-loaded chitosan oligosaccharide-oxidized hyaluronic acid composite nanogels for infected wound healing. Int J Biol Macromol 253(6): 127248.
- Pu S, Zhang J, Shi C, Hou X, Li K, et al. (2024) A multifunctional chitosan based porous membrane for pH-responsive antibacterial activity and promotion of infected wound healing. J Mater Chem B 12(29): 7191-7202.
- Wang X, Ren M, Wang N, Ling J, He Y, et al. (2024) Zeolitic imidazolate framework-8@polydopamine decorated carboxylated chitosan hydrogel with photocatalytic and photothermal antibacterial activity for infected wound healing. J Colloid Interface Sci 675: 1040-1051.
© 2025 Mohammad Reza Kasaai*. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






