- Submissions

Full Text
Journal of Biotechnology & Bioresearch
Development of Chloroplast Engineering in Plant
Peng Xu1, Chenxin Zhao1 and Shuying Feng1,2*
1Medical College, Henan University of Chinese Medicine, Zhengzhou, Henan 450046, China
2Henan Engineering Research Center for Chinese Medicine Foods for Special Medical Purpose, Zhengzhou, 450046, China
*Corresponding author:Shuying Feng, Medical College, Henan University of Chinese Medicine, Henan Engineering Research Center for Chinese Medicine Foods for Special Medical Purpose, Zhengzhou, 450046 China
Submission: July 08, 2025;Published: August 05, 2025

Volume5 Issue 5August 05, 2025
Abstract
Chloroplast engineering holds immense promise to address pressing global challenges in agriculture, health, and energy because chloroplast can produce bioactive compounds with potential pharmaceutical and nutritional value, making them valuable resources for modern society. Recently many research results reported advancements in chloroplast engineering in plants. However, it is still challenging to engineer chloroplast freely. In this review, we address chloroplast engineering development via nucleartargeted genetic engineering approaches and chloroplast-targeted genetic engineering approaches in plants. We summarize the new approaches to engineering chloroplast, discuss the current limitations and prospects of chloroplast engineering, and propose the possible applications of new technologies to engineer chloroplast.
Keywords:Chloroplast engineering; Gene editing; PPR, CRISPR/Cas; TALEN; Base-editing
Introduction
With the rapidly growing world population, global food, health, medicine, and energy crises have attracted extensive attention [1]. Because chloroplasts are involved in the production of bioactive compounds with potential pharmaceutical and nutritional value. For instance, some plants utilize chloroplasts to synthesize phytochemicals like flavonoids, alkaloids, and terpenoids. These compounds often possess antioxidant, antimicrobial, and anticancer properties, making them of significant interest for medical and nutritional research. So, engineering chloroplast has shown great significance in meeting the increasing demands of modern society. Chloroplasts play a crucial role in sustaining life on Earth and are essential photosynthetic organelles for some protists and plant cells [2]. The chloroplasts of all eukaryotic algae and all embryonic plants (bryophytes, ferns, and seed plants) are the product of a single endosymbiotic event that occurred about 1.5 billion years ago through the uptake of cyanobacteria by heterotrophic protists [3]. During the subsequent co-evolution with the nuclear genome, the chloroplast genome retained its independence [4], called plastid or ptDNA, typically contains only 100-250 genes (about 130 genes in seed plants) that play a key role in chloroplast development [2]. Chloroplast development was regulated by nuclear genes and ptDNA together. Because of the high copy numbers of chloroplast genes, chloroplast has been known as a powerful expression system to produce heterologous proteins on a large scale [5]. Previous reports not only indicate that chloroplasts could play additional major roles in human health and well‐being well beyond providing food, feed, fuel, and oxygen in the following era [6], but also show great potential for increasing crop production, producing drugs, and reducing the cost the of renewable biofuels [3].
With the development of Genome Editing (GE) technologies, like CRISPR-based GE methods, there are more chances to engineer chloroplast via genetic engineering on nuclear genome or ptDNA. Current reports showed that researchers can regulate chloroplast development by overexpressing or knocking out some genes in nuclear, or directly applying genome editing on ptDNA in chloroplast. The significance of chloroplast engineering leads many researchers to conduct numerous related studies. However, there is a lack of reviews on the development of chloroplast engineering. Moreover, the application of base editing offers a promising approach to engineering ptDNA directly to regulate gene expression in chloroplast. To gain a deeper understanding of chloroplast engineering, it is crucial to explore potential approaches for chloroplast engineer and enhance the application of chloroplast engineer. In this review, we discussed the current genetic engineering approaches (nuclear-targeted GE approaches and chloroplast-targeted GE approaches) to engineer chloroplast, specifically highlighting novel TALEN-based bas editing in chloroplast. Furthermore, we outline the primary challenges and future directions for further research to foster the development of chloroplast engineering.
Chloroplast engineering via nuclear-targeted genetic engineering approaches
The manipulation of plant genomes using current genome editing tools has the potential to increase crop quality and yield, thereby meeting the growing agricultural demands of the coming decades. To manipulate gene expression, except by overexpressing target genes via transformation, numerous genome editing tools, such as CRISPR-Cas, base editors, and prime editing tools, have been successfully developed and applied to plants. Nuclear genes and ptDNA both can affect chloroplast development, then regulate photosynthesis and secondary metabolites synthesized in chloroplast, like carotenoid biosynthesis, and lipid synthesis [7,8]. Therefore, engineering chloroplast by manipulating gene expression of nuclear genes or ptDNA has shown great potential to synthesize useful compounds and provide additional agricultural and medical benefits. In tomato (Solanum lycopersicum), Geranylgeranyl diphosphate (GGPP) produced by GGPP Synthase (GGPPS) serves as a precursor for many plastidial isoprenoids, including carotenoids. The three tomato GGPPS isoforms (SlG1, S1G2, and S1G3) played an important role in regulating carotenoid levels in the chloroplast. Knocking out these genes leads to decreased carotenoid levels in young leaves [9]. By contrast, knocking out a nuclear gene, the Non-phototropic seedling1 (Nps1) gene, can upregulate the expression levels of carotenogenesis pathway genes and increase carotenoid content in fruits [10]. In Lettuce (Lactuca sativa), researchers identified an LsNRL4 gene, which encodes an NPH3/RPT2-Like (NRL) protein and has a function that contributes to chloroplast development, photosynthesis and leaf angle. Overexpressing LsNRL4 significantly improved photosynthesis in the chloroplast [11]. In Brassica napus, BnaNTT1 binds ATP/ Adenosine Diphosphate (ADP), transports cytosolic ATP into the chloroplast, and exchanges ADP into the cytoplasm. Overexpress BnaNTT1 enhances the biosynthesis process in chloroplast and promotes seed oil accumulation [12]. These reports provide new approaches to engineer chloroplast.
However, it is very difficult to manipulate ptDNA directly in chloroplast. The use of CRISPR-Cas9, which is widely used for plant genome editing, has rarely been reported to show successful GE in chloroplast. This may be due to difficulties in transferring CRISPR/ RNPs into chloroplasts. Proteins must pass through translocons (TOC and TIC) to enter chloroplasts. However, proteins have to be denatured before entering these translocons. This suggests that it may be nearly impossible to transfer gRNA and Cas9 together into plant cells. A new genome editing technology called “Edit Plasmids” has been reported and has shown some positive results in Chlamydomonas reinhardtii chloroplasts [13]. Though this report claimed that they successfully delivered a fully functional expression cassette into the chloroplast to achieve genome editing, this method still requires further validation. It represents a new approach to chloroplast genome editing. Currently, there is no ideal CRISPR/Cas9 system for chloroplast genome editing in plants. However, researchers have found that using engineered TAL systems can efficiently edit genes in both chloroplasts and mitochondria [14,15]. A research group from South Korea has developed an efficient chloroplast base editor called DddA-derived Cytosine Base Editor (DdCBE). Their results show that the editing efficiency can reach 99% in regenerated plantlets and lettuce calli [14]. These studies demonstrate a novel approach to ptDNA GE to engineer chloroplast.
Chloroplast engineering via RNA binding protein
RNA binding proteins can affect RNA life activities, therefore affect gene expression in plant cells [16]. Among RNA-binding proteins, Pentatricopeptide Repeat (PPR) proteins have been well studied in the last decades. Though encoded by nuclear genes, almost all PPR proteins target and function in the chloroplasts [17]. By recognizing and binding specific sequences, PPR proteins can affect chloroplast gene expression and metabolic processes [18]. In 2016, the researcher designed and synthesized a series of artificial PPR proteins that can bind to specific sites of targe ssRNAs. They overserved the interactions between different PPR codes and RNA bases at the atomic level, and elucidated the molecular basis for the modular and specific recognition patterns of the RNA bases U, C, A, and G. This study shows a novel path for RNA manipulation technology. Recently researchers identified more PPR proteins that affect chloroplast development. In Arabidopsis, PPR287 played a crucial role by affecting transcript levels of chloroplast rRNAs, which then affect chloroplast biogenesis and function. These results indicate that PPR287 affects the stability of chloroplast rRNAs [19]. ORGANELLE TRANSCRIPT PROCESSING 970 (OTP970) plays a key role in RNA editing of ndhB transcripts at site 149 (ndhB-C149) in chloroplast [20]; DELAYED GREENING 409 (DG409) directly interacted with 2 DYW-type PPR proteins (EARLY CHLOROPLAST BIOGENESIS2 [AtECB2] and DYW DOMAIN PROTEIN2 [DYW2]) and 3 multiple organellar RNA editing factors (MORF2, MORF8, and MORF9), which indicate that DG409 is involved in RNA editing via protein complexes and is therefore essential for chloroplast development in Arabidopsis [21].
In rice (Oryza sativa), a PPR protein OsTHA8 affected the editing efficiency of ndhB-611/737 and rps8-182 transcripts in normal conditions. Further research indicated that OsTHA8 facilitates RNA editing by forming an editosome with multiple organellar RNA editing factors (OsMORF8) and thioredoxin z (OsTRXz), which function in RNA editing in rice chloroplasts. Therefore, the expression of PEP-dependent genes and photosynthesis-related genes can be regulated by PPR proteins OsTHA8 [22]. Another group found a PPR protein, which they named SSA1(seedling stage albino1), which is essential in RNA editing of ndhB-737 and intron splicing of atpF and ycf3–2 in chloroplast. The following research indicated that SSA1 physically interacted with two new RNA editing partners, OsMORF8 and OsTRXz, which have potential functions in RNA editing and chloroplast biogenesis [23]. A research team also found a P-type PPR protein OsPPR11 is necessary for ndhA, and ycf3-1 introns splicing and interact with CRM family protein OsCAF2, suggesting that these two proteins may form splicing complexes to regulate group II introns splicing. In the osppr11 mutant, photosynthetic complex accumulation decreased significantly. These results indicated that OsPPR11 is essential for chloroplast development and function by affecting group II intron splicing in rice [24]. Besides, a newly identified PPR protein, WAL3 (Whole Albino Leaf on Chromosome 3), plays an important role in plant chloroplast development. The WAL3 protein affected the splicing of multiple group II introns. Transcriptome sequencing showed that WAL3 is involved in multiple metabolic pathways including chlorophyll synthesis and photosynthetic-related metabolic pathways [25]. These reports revealed many new PPR proteins that are essential for chloroplast development in rice.
Currently, few dPPR in vivo experiments have been successful. In 2019, Barkan’s laboratory expressed dPPR proteins from nuclear transgenes to induce an approximately 40-fold increase in the expression of plastid foreign genes, the maximal protein accumulation of which was close to the Rubisco level [26]. In another experiment, Barkan’s laboratory successfully designed a dPPR protein in transgenic Arabidopsis plants and used it in genetic engineering research to bind a specific mRNA sequence in chloroplasts, demonstrating that the synthetic dPPR protein can reliably and selectively combine with targeted RNA in vivo [27]. In another case, Hammani et al. carried out a functional complementation experiment to show that synthetic dPPR protein binds to its expected mRNA target with specificity in vivo and successfully replaced a natural PPR protein by stably processing rbcL mRNA [27]. These results indicated that dPPR proteins can be artificially designed and modified to approximate the functions of natural PPR proteins and highlighted methods that can be used to regulate when, where, and to what extent chloroplast genes are expressed. Researchers can design more useful dPPR proteins with more identified PPR proteins and detailed binding sites revealed, that the growing repertoire of dPPR proteins with clear RNA binding sites represents tools to exploit the unique properties of the chloroplast gene expression system.
Chloroplast engineering via Base-editing on ptDNA
CRISPR/Cas9 genome editing tools face significant challenges in chloroplast applications due to the difficulty of delivering both guide RNA (gRNA) and the Cas9 protein to organelles, as well as simultaneously expressing these two components within organelles [14]. As a result, genome editing tools that do not require gRNA, such as Transcription Activator-Like Effector (TALE) nucleases, have shown greater promise for achieving chloroplast genome engineering. Recent advancements in CRISPR-free DddADerived Cytosine Base Editors (DdCBEs) have demonstrated the ability to facilitate targeted C∙G-to-T∙A base substitutions in mitochondrial DNA of mammalian cells, thereby suggesting the potential application of TALE-derived base editors in chloroplast engineering [28,29]. In 2021, a group of researchers from South Korea developed an efficient method for chloroplast base editing (C∙G-to-T∙A) to enhance the efficacy of chloroplast genome engineering. They devised a Golden Gate cloning system consisting of 424 modular TALE subarray plasmids and 8 expression vectors to construct a Ddda-derived cytosine base editor (DdCBE) plasmid for editing chloroplast genes. By employing the DdCBE to target the 16S rDNA in lettuce and rapeseed calli, they achieved a reasonably high editing frequency of 38% within the chloroplast. To achieve even higher base editing efficiency, they designed a novel DdCBE target in the 16S rRNA gene that conferred resistance to streptomycin and spectinomycin. Through antibiotic selection, the transformed protoplasts of lettuce yielded calli and plantlets with a 99% editing efficiency. Overall, the researchers developed a novel DdCBE system to enhance the efficiency of chloroplast point mutagenesis [14].
In addition to C-to-T editing, A-to-G editing also holds the potential for chloroplast DNA modification. In 2022, the same South Korean research team reported a successful generation of heritable homoplasmic A-to-G edits in ptDNA, leading to phenotypic changes. They constructed an expression vector comprising Transcription Activator-Like Effector (TALE)-linked deaminases (TALEDs), which included custom-designed TALE DNA-binding arrays, split DddAtox, and an engineered deoxyadenosine deaminase (TadA- 8e). Co-transfecting in vitro transcripts (mRNA) encoding TALEDs with a Plastid Transit Peptide (PTP) into lettuce protoplasts resulted in A-to-G editing frequencies reaching 46%. Furthermore, the expression of TALEDs facilitated nearly complete (~99%) adenine editing in the T1 generation. Collectively, their findings demonstrated that base edits induced by TALEDs in ptDNA could be transmitted to subsequent generations [29]. In 2023, the team led by Gao Caixia further advanced the development of a new modular base editing system, CyDENT, which edits cytosine bases on the bottom strand of the target DNA, resulting in phenotypic changes. The system consists of TALE protein, FokI incision enzyme, single strand specific cytosine deaminase, DNA exonuception enzyme and UGI. The researchers transformed rice protoplasts with cpCyDENT-L (harboring a FokI-Lnickase) and cpCyDENT-R (harboring a FokIRnickase) with TALE proteins targeting the endogenous ribulose- 1,5-bisphosphate carboxylase-oxygenase (RuBisCO) large subunit gene (rbcL). It was observed that when the rbcL target was treated with cpCyDENT-L, the editing efficiency was low but detectable, and only the cytosine bases on the bottom strand of the target DNA were edited, with a frequency of about 1.67% at G1. Therefore, these results highlight cpCyDENT’s ability to selectively and precisely base edit DNA strands in the chloroplast genome [30].
In the same year, another group adopted two different strategies to construct Tale-ABE, one by assembling Tale-ABE using a single tale array of a Bipartite Nuclear Localization Sequence (bpNLS), nN-terminal TALE domain, the RVD repeat region, C-terminal TALE domains, and functional domains, and functional domains to perform the base editing, termed single TALE-ABE (sTABE). The other is to use a pair of TALE-ABE, naming them Pairs TALE-ABE (pTABE). To simply build different base editor designs, individual parts are built as modular clone (MoClo) modules and assembled using Golden Gate clones. The editor pTABE_v6 mediates the transformation of rice chloroplast OspsaA gene A•t to g•C, with an editing frequency of up to 98%. The study further analyzed 150 bp regions on both sides of TALE binding sites in albino lines and unexpectedly found several A•T-to-G•C mutations with different conversion frequencies in these regions, suggesting that pTABE_v6 can induce off-target editing of the chloroplast genome. By analysis no mutant strains were verified in the absence of UGI, no DDDA-induced editing of C•G-to-T•A, C•G-to-T•A changes were found. These results suggest that pTABE_v6 can produce almost homogeneous A•t to g•C editing in rice chloroplasts, despite the presence of off-target editing [31]. The DdCBE and TALEDs base editors represent groundbreaking advancements in targeted editing of chloroplast DNA. These novel editing methods offer new avenues for engineering chloroplasts through ptDNA base editing.
Summary and Outlook
Chloroplast engineering represents a dynamic field with vast potential across diverse sectors. Chloroplast can be engineered through nuclear genome genetic engineering and ptDNA genome modification. Chloroplast is rich in energy, pigments, and raw materials, and involved in many significant progresses in developing plants with resistance to various stresses, phytoremediation of toxic metals, and production of vaccine antigens, biopharmaceuticals, biofuels, biomaterials, and industrial enzymes, which makes them ideal biological factories [32]. Therefore, it has the potential for various applications in plant biotechnology via chloroplast engineering. In this review, we summarized current chloroplast engineering applications and suggested potential strategies that can be applied to chloroplast engineering in the future (Figure 1). Nuclear genes play a key role in chloroplast engineering. Many proteins, which are expressed from nuclear genes, can play essential roles in many important synthesis pathways in chloroplast. More research needs to be conducted to reveal the detailed mechanism and gene regulation pathway. It is possible to achieve RNA editing in chloroplast via PPR proteins with specific sequences. Many new PPR genes have been identified recently and show functions involved in the gene expression process in chloroplast [33]. These results will help researchers to design new tools for chloroplast engineering.
Figure 1:Summary of the current genetic engineering approaches to engineer chloroplast development. Chloroplast engineering can be conducted via nuclear-targeted genetic engineering and chloroplast-targeted genetic engineering. Nuclear gene manipulation can affect chloroplast development in many ways, like regulating related synthesis pathways or affecting mRNA translation in chloroplast. The TALEN-based base editor can directly manipulate ptDNA to engineer chloroplast.
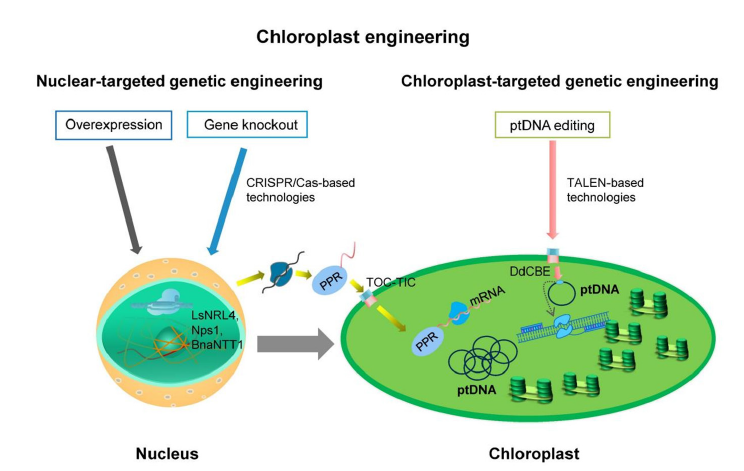
To efficiently utilize these genes for engineering chloroplast, genetic engineering approaches are important. Therefore, genome editing tools and transformation technologies are necessary for chloroplast engineering. Currently, genome editing tools and transformation technologies are mainly used for nuclear genome engineering, like CRISPR-Cas base genome editing tools, PEGmediated transformation, Agrobacterium-mediated delivery, biolistic-mediated delivery, etc [3]. Nuclear genetic engineeringbased chloroplast engineering approaches can benefit from these transformation methods. However, it is very difficult to engineer the chloroplast genome directly due to the lack of chloroplast-targeted transformation technologies. CRISPR/Cas-based genome editing tools are difficult to apply on ptDNA and face challenges due to CRISPR/RNPs not transferring into chloroplasts [14]. A promising solution is to deliver CRISPR/Cas expression cassette or CRISPR/ RNPs directly into chloroplast. To achieve it, a proper biolistic protocol is necessary because chloroplasts are too small to target. because of multiple copies of ptDNA in the chloroplast, CRISPR/ Cas expression cassette delivery is much more effective to engineer chloroplast. Novel vectors to express CRISPR/Cas protein and gRNA need to be constructed. To avoid using CRISPR/Cas-based genome editing tools, novel TALEN editing tools, designed by researchers, can achieve high-efficiency ptDNA genome editing to engineering chloroplast [14,29,30]. These approaches showed great potential to engineer chloroplast and are worth future research to broaden ptDNA-targeted genome editing tools using TALEN-based genome editing tools. Another potential way to achieve genome editing on ptDNA is using Zinc Finger Proteins tools. However, it is very challenging to design proper expression vector and the editing efficiency need to be improved because previous reports have shown ZFN have lower editing efficiency compared to CRISPR/ Cas-based genome editing tools and TALEN-based editing tools. Chloroplast-targeted nanotube-based transformation technology also showed potential to achieve ptDNA editing in chloroplast [34], but still need more research results to provide solid proof for future application on chloroplast engineering. In summary, chloroplast engineering holds promise for various applications in plant biotechnology, and ongoing research is focused on overcoming challenges and expanding its potential. With further advancements, chloroplast engineering may revolutionize the production of valuable compounds and contribute to sustainable agriculture and environmental remediation.
Author Contribution
All authors took part in writing, reviewing, and editing the manuscript. P Xu wrote the manuscript and modified the paper. All authors reviewed the manuscript and approved it for publication.
Declaration of Competing Interest
The authors declare no conflicts of interest in relation to this research and its publication.
Availability of Data and Materials
All data of this article are included within the article. It can also be requested from the corresponding author or first author.
Ethical Approval
This article does not contain any studies with human participants performed by any of the authors.
Acknowledgement
This work was supported by the National Natural Science Foundation of China [U1804112], the Natural Science Foundation of Henan Province (232300421164), the Basic Research Project of the Key Research Program of Colleges and Universities in Henan Province (23ZX005), and the Zhongjing Core Scholar’s Research Initial Fund of Henan University of Chinese Medicine [00104311- 2023].
References
- Zhu HC, Li C, Gao C (2020) Applications of CRISPR-cas in agriculture and plant biotechnology. Nat Rev Mol Cell Biol 21(11): 661-677.
- An Y, Wang Y, Wang X, Xiao J (2022) Development of chloroplast transformation and gene expression regulation technology in land plants. Front Plant Sci 13: 1037038.
- Bock R (2015) Engineering plastid genomes: methods, tools, and applications in basic research and biotechnology. Annu Rev Plant Biol 66: 211-241.
- Zhang Y, Tian L, Lu C (2023) Chloroplast gene expression: Recent advances and perspectives. Plant Commun 4(5): 100611.
- Oey M, Lohse M, Kreikemeyer B, Bock R (2009) Exhaustion of the chloroplast protein synthesis capacity by massive expression of a highly stable protein antibiotic. Plant J 57(3): 436-445.
- Daniell H, Jin S, Zhu XG, Gitzendanner MA, Soltis DE, et al. (2021) Green giant-a tiny chloroplast genome with mighty power to produce high-value proteins: History and phylogeny. Plant Biotechnol J 19(3): 430-447.
- Hölzl G, Dörmann P (2019) Chloroplast lipids and their biosynthesis. Annu Rev Plant Biol 70: 51-81.
- Quian-Ulloa R, Stange C (2021) Carotenoid biosynthesis and plastid development in plants: The role of light. Int J Mol Sci 22(3): 1184.
- Barja MV, Ezquerro M, Beretta S, Diretto G, Florez-Sarasa I, et al. (2021) Several geranylgeranyl diphosphate synthase isoforms supply metabolic substrates for carotenoid biosynthesis in tomato. New Phytol 231(1): 255-272.
- Kilambi HV, Dindu A, Sharma K, Nizampatnam NR, Gupta N, et al. (2021) The new kid on the block: A dominant-negative mutation of phototropin1 enhances carotenoid content in tomato fruits. Plant J 106(3): 844-861.
- An G, Qi Y, Zhang W, Gao H, Qian J, et al. (2022) LsNRL4 enhances photosynthesis and decreases leaf angles in lettuce. Plant Biotechnol J 20(10): 1956-1967.
- Hong Y, Xia H, Li X, Fan R, Li Q, et al. (2022) Brassica napus BnaNTT1 modulates ATP homeostasis in plastids to sustain metabolism and growth. Cell Rep 40(2): 111060.
- Yoo BC, Yadav NS, Orozco Jr EM, Sakai H (2020) Cas9/gRNA-mediated genome editing of yeast mitochondria and Chlamydomonas chloroplasts. PeerJ 8: e8362.
- Kang BC, Bae SJ, Lee S, Lee JS, Kim A, Lee H, Get al. (2021) Chloroplast and mitochondrial DNA editing in plants. Nat Plants 7(7): 899-905.
- Cho SI, Lee S, Mok YG, Lim K, Lee J, et al. (2022) Targeted A-to-G base editing in human mitochondrial DNA with programmable deaminases. Cell 185(10): 1764-1776.e12.
- Glisovic T, Bachorik JL, Yong J, Dreyfuss G (2008) RNA-binding proteins and post-transcriptional gene regulation. FEBS Lett 582(14): 1977-1986.
- Gully BS, Cowieson N, Stanley WA, Shearston K, Small ID, et al. (2015) The solution structure of the pentatricopeptide repeat protein PPR10 upon binding atpH RNA. Nucleic Acids Res 43(3): 1918-1926.
- Barkan A, Small I (2014) Pentatricopeptide repeat proteins in plants. Annu Rev Plant Biol 65: 415-442.
- Lee K, Park SJ, Han JH, Jeon Y, Pai HS, et al. (2019) A chloroplast-targeted pentatricopeptide repeat protein PPR287 is crucial for chloroplast function and Arabidopsis development. BMC Plant Biol 19(1): 244.
- Fu M, Lin X, Zhou Y, Zhang C, Liu B, et al. (2022) OTP970 is required for RNA editing of chloroplast ndhB transcripts in Arabidopsis thaliana. Genes (Basel) 13(1): 139.
- Wang H, Liu J, Zhao W, Terzaghi W, Deng L, et al. (2023) DELAYED GREENING 409 encodes a dual-localized pentatricopeptide repeat protein required for chloroplast and mitochondrial development. Plant Physiol 192(4): 2768-2784.
- Wang Y, Duan Y, Ai P (2023) OsTHA8 encodes a pentatricopeptide repeat protein required for RNA editing and splicing during rice chloroplast development. The Crop Journal 11(5): 1353-1367.
- Wang Y, Yang Z, Zhang M, Ai P (2022). A chloroplast-localized pentatricopeptide repeat protein involved in RNA editing and splicing and its effects on chloroplast development in rice. BMC Plant Biol 22(1): 437.
- Zhang Q, Chen C, Wang Y, He M, Li Z , et al. (2023) OsPPR11 encoding P-type PPR protein that affects group II intron splicing and chloroplast development. Plant Cell Rep 42(2): 421-431.
- Lv Y, Wang Y, Zhang Q, Chen C, Qian Q, et al. (2022) WAL3 encoding a PLS-type PPR protein regulates chloroplast development in rice. Plant Sci 323: 111382.
- Rojas M, Yu Q, Williams-Carrier R, Maliga P, Barkan A (2019) Engineered PPR proteins as inducible switches to activate the expression of chloroplast transgenes. Nat Plants 5(5): 505-511.
- McDermott J, Watkins KP, Williams-Carrier R, Barkan A (2019) Ribonucleoprotein capture by in Vivo expression of a designer pentatricopeptide repeat protein in Arabidopsis. Plant Cell 31(8): 1723-1733.
- Lei Z, Meng H, Liu L, Zhao H, Rao X, et al. (2022) Mitochondrial base editor induces substantial nuclear off-target mutations. Nature 606(7915): 804-811.
- Mok YG, Hong S, Bae SJ, Cho SI, Kim JS (2022) Targeted A-to-G base editing of chloroplast DNA in plants. Nat Plants 8(12): 1378-1384.
- Hu J, Sun Y, Li B, Liu Z, Wang Z, et al. (2023) Strand-preferred base editing of organellar and nuclear genomes using CyDENT. Nat Biotechnol 42(6): 936-945.
- Zhang D, Boch J (2023) Development of TALE-adenine base editors in plants. Plant Biotechnol J 22(5): 1067-1077.
- Adem M, Beyene D, Feyissa T (2017) Recent achievements obtained by chloroplast transformation. Plant Methods 13: 30.
- Wang X, An Y, Xu P, Xiao J (2021) Functioning of PPR proteins in organelle RNA metabolism and chloroplast biogenesis. Front Plant Sci 12: 627501.
- Kwak SY, Lew TTS, Sweeney CJ, Koman VB, Wong MH, et al. (2019) Chloroplast-selective gene delivery and expression in planta using chitosan-complexed single-walled carbon nanotube carriers. Nat Nanotechnol 14(5): 447-455.
© 2025 Shuying Feng. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






