- Submissions

Full Text
Journal of Biotechnology & Bioresearch
The Elixir of Cistus ladanifer: Delving into its Chemical Complexity and Therapeutic Deeds
Kaoutar Bouothmany1,2, Boutaina Addoum1*, Mohammed Bourhia3, Fouad Mellouki2, Farid Khallouki4, Mohammed El Mzibri1, Mohammed Attaleb1 and Laila Benbacer1
1Biology and Medical Research Unit, National Center for Energy, Sciences and Nuclear Techniques CNESTEN, Morocco
2RU Microbiology, Biomolecules and Biotechnology, Laboratory of Chemistry-Physics and Biotechnologies of Biomolecules and Materials, FST Moham Media, Hassan II University, Casablanca 28806, Morocco
3Department of Chemistry and Biochemistry, Faculty of Medicine and Pharmacy, Ibn Zohr University, Laayoune, Morocco
4Biology Department, FSTE, Moulay Ismail University of Meknes, BP 609, Errachidia 52000, Morocco
*Corresponding author:Addoum Boutaina, Biology and Medical Research Unit, National Center for Energy, Sciences and Nuclear Techniques CNESTEN, Morocco
Submission: May 29, 2024;Published: June 21, 2024

Volume5 Issue3June 21, 2024
Abstract
Cistus ladanifer, known as the Crimson-Spot Rockrose, thrives in the Mediterranean’s harsh conditions, captivating researchers with its pharmacological promise. This review compiles existing pharmacological data on C. ladanifer, examining its chemical composition, diverse applications and biological activities. With implications for pharmaceuticals, cosmetics and agri-food industries, understanding C. ladanifer’s potential offers opportunities for innovation and discovery.
Keywords:Cistus ladanifer; Crimson spot rockrose; Chemical composition; Pharmaceutical applications; Cosmetic applications, Pharmacological potential
Graphical Abstract
Graph 1:
Introduction
Since ancient times, humans have depended on natural resources derived and isolated from plants for different applications. For a long time, people have used medicinal plants to make drugs. There are 12 drug making recipes that list over 250 different plants [1]. Interest in medicinal plants has continued for more than 1,000 years [2] and in recent years, intense research has been carried out for isolation and pharmacological characterization of various natural sources. Treatment with medicinal plants in optimal use is considered safe since there are few if any side effects, which is the biggest advantage. In fact, according to the World Health Organization, about three quarters of the world’s population currently uses various forms of traditional medicines to treat illness. The importance of traditional medicine is due to the ability of medicinal plants to synthesize secondary metabolites of various chemical and biochemical structures including alkaloids, glycosides, terpenes and polyphenols, which are responsible for physiological functions in plants, as well as several biological properties and therapeutic cures [3]. Secondary metabolites have been proven to act as pharmacological targets, most of the common drugs that are believed to be obtained from plant extracts are atropine, vinblastine, artimesinin, tubocurarine, digoxin, pilocarpine, quinidine, physostigmine, morphine, reserpine, ephedrine, taxol, colchicine, vincristine, quinine and aspirin [4]. Therefore, scientists are continuously screening natural resources, including medicinal plants, by exploring the bioavailability of therapeutic compounds in unexplored plant species.
Cistus plants, which are also called rockroses, are native to the Mediterranean. They are a genus of evergreen shrubs in the family Cistacea [5]. Antioxidant, antibacterial, anticancer, antiinflammatory and other pharmacological proprieties of the family Cistaceaare are well documented [6]. Cistus species are spread over the Mediterranean region, in which each zone is colonized by different species, depending on climatic and soil conditions [5]. As herbal tea infusions or extracts, they have traditionally been used in Mediterranean folk medicine for treatment of inflammatory and cancer-related disorders [7,8]. Cistus ladanifer (CL) is currently the subject of recent research in various fields pharmaceutical, cosmetic, perfume and food industries, and is widely used in herbal medicine due to its physiological activities. It is an underestimated natural resource known for the presence of various compounds responsible for its therapeutic proprieties. Several pharmacological studies have shown that extracts of CL can reduce inflammation, ease pain, and stop spasms [9]. Antibacterial and antifungal potential of extracts of CL were described [6,7,10-12]. However, there are few reports describing the cytotoxic potent of this plant [7,12-14]. The existence of bioactive compounds with a variety of biological activities was discovered using chromatographic and spectroscopic investigations. Tetraterpenoids such as labdanoids, diterpenoids, and phenolic chemicals such as flavonoids and tannins are all essential components of phytochemicals [15]. The present work reviewed available scientific data on CL, focusing on its biological activities, chemical composition, as well as applications and uses.
Genus cistus
The Cistaceae are an indigenous Mediterranean family of almost 200 species of shrubs and 8 genera including Cistus. The plants of this family are fragrant and are therefore used in perfumery and for ornamental purposes. In addition, plants in the family Cistaceae are pyrophilic, and adapted to fires in Mediterranean forests, which destroy large areas of forest, moreover, their seeds resist and are characterized by seasonal reproduction [16]. There are three subgenera in the genus Cistus “Cistaceae”: Cistus, Leucocistus, and Halimioides. The first one has purple-flowered species, and the last two have white flowered species [5].
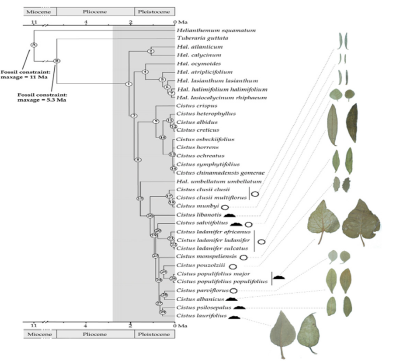
Cistus plants, belonging to the genus Cistus, are characterized by their perennial nature, evergreen foliage, and simple, usually opposite, 2-8cm shrubby growth. Their flowers, which can be found either individually or in loose clusters, are perennial and boast three or five sepals that oppose the five petals. The petal color varies, ranging from white, purple, to dark pink, depending on the subgenus, with some species exhibiting a dark red spot at the base of each petal. The fruits, capsules on erect stems, are separated by 5 to 10 valves. Trichomes, in the form of star-shaped or simple hairs, as well as glands of various shapes, adorn all Cistus leaves. These leaves secrete a resinous exudate known as Labdanum in varying amounts due to the presence of secretory glands Haut du formulaire [5,6,17] (Figure 1).
Figure 1:Phylogenic three of cistus based on the investigation of Guzman et al (Guzmán et al., 2009).
Distribution and traditional use
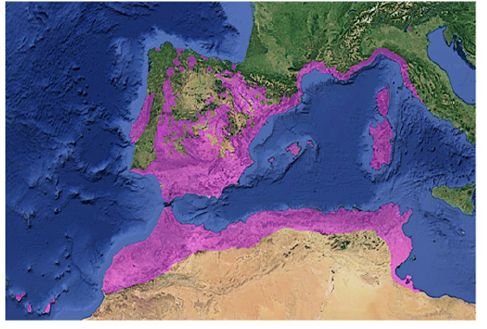
C. ladaniferus is widespread throughout the Mediterranean region, from Morocco to Portugal to the Middle East [Portugal, Spain, France, Italy, Sicily, and the northern regions of Algeria and Morocco], as well as on the islands Canary Islands. In Morocco C. ladaniferus is common in the Kenifra and Rif regions (Figure 2) [18]. Indeed, the Mediterranean region is extremely rich in natural resources and presents great biological diversity. C. ladaniferus is a predominant species in this region, well adapted to the Mediterranean climate and possibly to environmental changes, it resists severe drought and infertile and highly degraded soils [19]. Cistus ladaniferus has been frequently used in traditional medicine for a very long time. By a simple decoction of their leaves, it is used in the treatment of various diseases such as an antidiarrheal, antiinflammatory, antacid, antispasmodic, anti-tumor and vasodilator agent [18]. In traditional medicine, CL is exploited in the form of infusions, extracts and resin for very diverse purposes, such as antiinflammatory, anti-ulcerogenic, healing, spasmolytic, antimicrobial, cytotoxic or vasodilator remedies, it is also used as an antidiarrheal, antacid and antispasmodic. Labdanum is traditionally used in low doses as an ingredient in sedative tea, coffee and other infusions to prevent insomnia and anxiety [7,8,15].
Figure 2:Distribution of Cistus ladanifer in Spain and other southern areas according to GBIF (Global Biodiversity Information Facility)(Mediavilla et al., 2021).
Botanical description
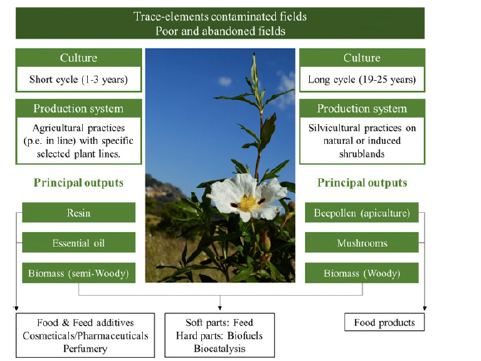
CL, or rock rose is an aromatic, perennial, and herbaceous plant, belonging to the family Cistaceae, native to the Mediterranean region [6]. In northern Morocco, it is called “Touzal,” and in other parts of Morocco, it is called “Argale.”. In traditional medicine, due to its pharmacological properties, it is used as general remedies (Figure 3) [19]. CL is found anywhere in the Mediterranean region, including Portugal, Spain, France, Italy, Sicily and northern regions of Algeria and Morocco, as well as on the Canary Islands [18,19]. In Morocco, CL is common in the Kenifra and Rif regions. CL occurs in the form of a perennial shrub, the height of which is from 2m to 3m, the leaves are green, large with a diameter of 5-10cm, lanceolate, flowers consist of five petals which may have a reddish spot at the base of each petal or be completely white. Two types of trichomes are present in the leaves of CL: The secretory, having multicellular structures in the form of small heads with a short peduncle of 20 to 30μm secreting an oleoresin called the Labdanum, and the nonsecretory, which are unicellular hairs stellate with 6-20 branches [18]. The fruits are globular capsules made of lignin that are resistant to harsh environmental conditions and have seven to ten compartments [20].
Figure 3:A schematic representation of the different area of use of citrus lodanifer [21].
Usage and applications of CL
Perfumery: The labdane present in CL has a typical scent of deep, sweet, balsamic, lingering smell and it is therefore used to make perfume, cosmetics, soaps, and cleaning products as a natural fixative, a fragrance, and as an incense [21-23]. It is one of the most important parts of some high-quality perfumes, mostly those for men [21]. The sesquiterpene compounds of the Ledol and Ambroxide families present in CL essential oils and the products resulting from oxidative degradation of labdanum-type diterpenes are responsible for the typical smell of CL [5,23,24]. Furthermore, diterpene compounds are responsible for prolonged fragrance of Labdanum and they are also good replacements for ambergris, which comes from a protected wild source called the cachalot Physetercatodon and is a valuable raw material for the perfume industry [21,25,26]. The perfume industry uses both labdanum and essential oil from CL, which constitute 30% of modern perfumes with excellent fixative properties [5,27,28].
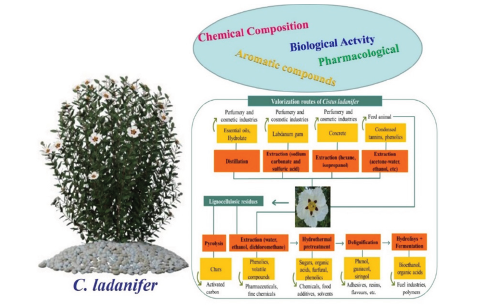
Cistus ladanifer as a potential feedstock for biorefineries
According to a recent study conducted in 2023, rockrose is a widespread shrub species in the Mediterranean region well known due to its production of labdanum gum, especially in the hot season. Its leaves and branches can be subjected to different extraction and distillation processes to produce various types of extracts (Figure 4). The natural extracts of C. ladanifer have several applications, especially in the perfumery and cosmetics sector. C. ladanifer extracts, in addition to presenting interesting odoriferous properties, are also known for their bioactive properties, such as antioxidant and antimicrobial. Use of this species in animal feed or phytostabilisation of mining areas has also been successfully applied. On the other hand, the lignin and polysaccharides that are the major fractions from Cistus residues can be relevant sources of high-value products in a biorefinery framework. Recently, it has been reported that the residues obtained from the essential oil industry can sustain production of significant amounts of other marketable products, namely phenolic compounds, oligomeric and monomeric sugars, lignin, and lactic acid. As reported in Figure 5, the potential of C. ladanifer as a raw material to be fully valued in a biorefinery context, contributing to important revenues and generating an associated marketable biobased product portfolio [14].
Figure 4:Main conventional aromatic extracts obtained from C. ladanifer and respective extraction methods [14].
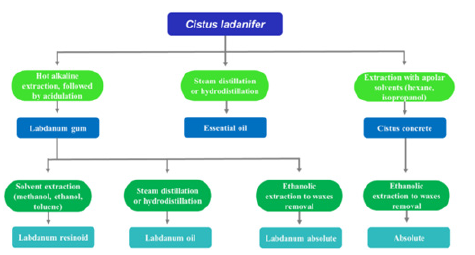
Figure 5:Potential path for use of C. ladanifer with an integrated valorization in a biorefinery framework for obtaining different added-value products [10].
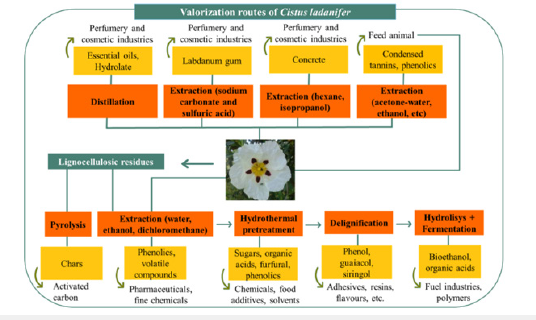
Food
CL is used as an antimicrobial preservative agent, also as a food additive [29]. Essential oils, and extracts of CL possess significant antioxidant activity [7,27,30] and therefore present a preservative effect of food oxidation and lipid peroxidation [31]. Moreover, seeds of CL are composed of 78.4% by tricylglycerides, mainly linoleic acid, that are used in small amounts to make tea, coffee, and other drinks [7,21]. Adding CL to food reduces the amount of toxic synthetic chemical additives [5]. Fatty acids, such as tocopherol [vitamin E], ascorbic acid [vitamin C], and reducing sugars [fructose and glucose] in the leaves and stems of CL, make the plant’s health benefits better [32]. Due to its remarkable nutritional value, bee pollen has been consumed for centuries; However, due to the presence of Labdanum, which is reported to be neurotoxic, hepatotoxic, and nephrotoxic, and therefore its consumption can be limited and used with caution in small amounts in tea, coffee, and other sedative teas [5].
Chemical composition
The phytochemical composition of CL has been characterized and reported previously, showing that this plant is rich in organic compounds belonging to various classes of secondary metabolites and responsible for the various therapeutic activities of the plant. The secondary metabolites differ according to the ecological and harvesting conditions of the plant, to the part of the plant and to the conditions of its preparation.
Chromatographic and spectroscopic analyses showed that large amounts of phenolic compounds were identified. These compounds play a major protective role for the plant from various stresses, whether biotic or abiotic [33]. In fact, solar irradiance [UV] and hydric stress [drought] increase the secretion of phenolic compounds mainly methylated flavonoids [34,35]. Furthermore, this class of secondary metabolites has traditionally been associated with antioxidant, antimicrobial or cytotoxic activities [7]. Several phenolic acids, including gallic and ellagic acids were identified in CL [10,13-16]. Gallic acid was found in leaves collected in Spain [16], phenolic acids derivatives have also been detected in aerial parts of CL. The presence of tannins, primarily punicalagin gallates, punicalagins and punicalins has been reported [7,15,16,18,32]. Ellagitannins and condensed tannins were revealed to be the most abundant compounds found in CL leaves and stems [7,10,16,36]. According to reports, the oleoresin that is secreted by the plant contains a large amount of flavonoids, known as aglycones. These flavonoids include apigenin, kaempferol, and their methylated derivatives, most notably 3-methyl-kaempferol and 3,7-dimethylkaempferol, as well as 3,4′-dimethyl-kaempferol,3,7,4′-trimethylkaempferol, 4′-O-methyl-apigenin,7-O-methyl-apigenin, and 7,4′ dilapigenin [34,37,38]. It was discovered that the flavonoids flavones, flavonols, flavonoid derivatives and flavon-3-ols were the only flavonoids found in CL together with kaempferol and its derivates [16,39]. Myricitin was also found in CL extracts [13].
In addition to phenolic compounds, CL is known for the presence of terpenes, mainly in labdanum and Cistus oils. In total, 72 terpenes, including 47 monoterpenes, 18 sesquiterpenes, and 7 labdane-type diterpenes, have been found in CL [15]. They were categorized as oxygenated sesquiterpenes, oxygenated monoterpenes, linear ester, monoterpenes, and sesquiterpenes [11]. In a different study the percentage of these components were put into three groups: oxygenated hydrocarbons, oxygenated sesquiterpenes, and monoterpenic ester [40]. Several factors such as diurnal, seasonal, ecological, drought, temperature and plant age, influence the production of these volatile compounds in Cistus. The class of monoterpenes were found in Cistus mainly in essential oils. In CL, the most abundant compounds include α-pinene, β-Pinene, Limonene, borneol, p-Cymene and camphene [5]. Oxygenated monoterpenes were also found in CL like Terpineol, Pinocarveol and Terpineol acetate [30,41,42]. The majority of terpenes found in essential oils of CL grown in northern Portugal are monoterpenes [23,28,43], Spain [38], Morocco [30] and south France [15]. In fact, chemical analysis of Cistus preparations showed the presence of sesquiterpenes. Aerial parts and essential oils of CL in in France, Portugal, Spain and Morocco were found to be rich in high concentrations of sesquiterpenes with vidiflorol being the most abundant. Furthermore, sesquiterpenes including δ-Cadinene, Aromadendrene, ledol are the most numerous and prevalent constituents found in CL essential oils [30,43].
All Cistus species secrete a gum, the labdanum, which is very fragrant and composed mainly of terpenes, in particular diterpenes of the labdan type [6,17]. The manoyloxide type is the most abundant diterpene found in CL [5,15]. CL from Spain is a source of diterpene compounds, such as 6-acetoxy-7-oxo- 8-labden-15-oic acid, 7-oxo-8-labden-15-oic acid, and oxocativic acid [38], while there were more labdane-type diterpenes in other CL from France, Portugal and Spain [23,44]. The diterpernes compounds protect plants from herbivores and environmental stresses like lack of water and UV light. It is also thought to be an allelopathic agent that could slow plant growth by stopping the roots and cotyledons from growing and making it harder for plants to grow in their natural environment [21,35].
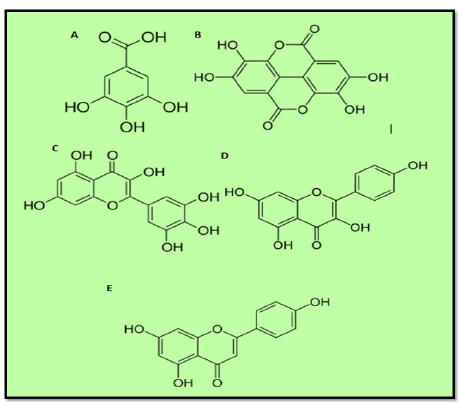
CL essential oils were found to have varying compositions according to the plant’s location. Viridiflorol, bornyl acetate, and camphene were the most prominent components in the CL leaf essential oil from Northern Morocco [45]. The compound 2,2,6-trimethylcyclohexanol, 4-terpineol, and α-pinene were all found in high concentrations in CL from Eastern Morocco, according to Zidane [30]. In France, Pinene, viridiflorol, ledol, and bornyl acetate, were found in the oil extracted from the leaves and stems of CL from Spain [46]. Essential oil from Algerian CL was found to be rich in 5,7-diamino-7,6-eudesmol, borneol, camphene, and δ-cadinene, with smaller amounts of eudesmol and 4-terpineol [47]. The chemical analysis of CL grows in Portugal poseesessesquiterpenes alcohols, such as viridiflorol [13.6-17.4%], globulol [3.1-5.0%], and an unknown sesquiterpene [2.7-6.0%], as well as diterpene alcohol 15-nor-labdan-8-ol [1.7-5.2%] [23]. The oxygenated sesquiterpene fraction was composed of viridiflorol [13.59%] and ledol [4.36%] [11] (Figure 6).
Figure 6:Major compounds found in C. ladaniferus, A : Gallic acid; B: Ellagic acid; C: Myricitin; D: kaempferol ; E: Apigenin.
Biological activities Antimicrobial activity
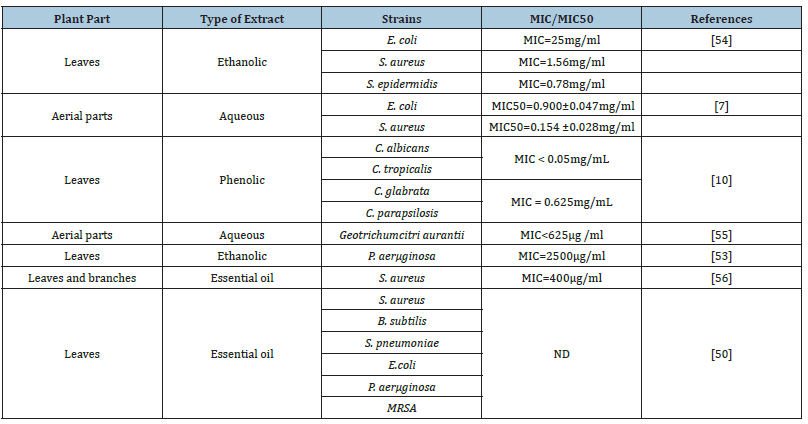
Widespread and sometimes inappropriate use of synthetic antibiotics is causing a therapeutic failure linked to the appearance and development of antibiotic resistance. CL is one of the plants that have been studied for bioactive molecules with antimicrobial activities on a variety of microorganisms, including Gram-positive bacteria, including S. aureus, Baecalis, and E. faecalis, Gram-negative bacteria, including E.coli, Klebsiellapneumoniae, P. aeroginosa, A. baumannii and H. pylori, yeasts, including C.tropicalis, C.albicans and C. parap, B. cinerea and G. citri-aurantii [6,10,21,39]. Extract of CL was more effective against E. faeciumand B. Subtilis. Additionally, CL phenolic extract had strong activity against Candida [33]. In addition, aqueous extract of CL has, in some cases, an antimicrobial effect as effective as the antibiotic neomycin [7].
Essential oils from CL were also studied for their antimicrobial activity and found to have an important activity against bacteria, including S. aureus, S. Typhi, E. coli, and A. Baumannii, fungi, including C. Tropicalis, C. Neoformans, R. Rubra, Penicillium sp., C. dubliniensis and C. glabrata [11,12,40]. Essential oils of CL exhibited strong activity compared to the antibiotic against A. Baumannii, indicating a good and promising effect [11]. In the same study, MIC and MBC results showed that essential oil of CL is bactericidal. The essential oil of CL has been shown to have antibacterial action against fungi such as A. niger, B. cinerea, Mucorracemosus and V. alboatrum, grampositive and negative pathogens of clinical relevance such as S.aureus, E. coli, S. pneumonia, P. aeruginosa, E. aerogenes and C. jejuni [48-50]. The fungi M. racemousis sensitive to essential oil obtained from CL due to the presence of sesquiterpenes compounds [51]. Moreover, Moroccan CL essential oil also has strong activity against multi-resistant Staphylococcus aureus, gram-positive bacterial strains with a minimum inhibitory concentration MIC of 50μg/ml and against Yersinia enterocolitica gram-negative bacterial strains with a MIC of 62.5μg/ml, also, activities against Staphylococcus epidermis, Streptococcus sanguinis, Staphylococcus aureus Pseudomonas aerμginosa, Yersinia enterocolitica and Acinetobacter baumannii [52].
Table 1:Antimicrobial activity of C. ladanifer.
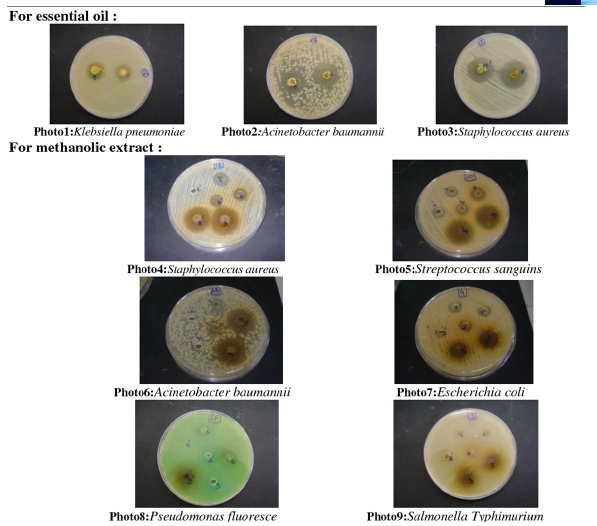
In Oulmes, situated in the middle Atlas region, the local populace employs Cistus ladaniferus for treating a spectrum of ailments. This particular species finds widespread application in numerous traditional medicinal practices due to its noted antimicrobial, antiviral, antitumor, anti-inflammatory, gastric, and antioxidant attributes. Encouraged by these aforementioned findings, Benyad [52] embarked on a deeper investigation into the antibacterial properties of the essential oil and various extracts (including hexane, dichloromethane, ethyl acetate, methanol, and aqueous extracts) derived from Cistus ladaniferus sourced from Oulmes, Morocco, against 14 microorganisms. Their results indicate that the essential oil exhibits strong antibacterial activity against both gram-positive and Gram-negative bacteria, with a preference for Gram-positive strains. Notably, the essential oil derived from Cistus ladaniferus demonstrated the highest zone of inhibition against Multi-resistant Staphylococcus aureus (28mm) and Staphylococcus aureus (Figure 7). Conversely, the lowest zone of inhibition was observed against Salmonella enteritidis and Pseudomonas aeruginosa (12mm). Particularly significant outcomes were noted for dilutions approximately at the 1/2 ratio.
Figure 7:Agar-well diffusion assay Zone of inhibition of growth of selected strains [52].
Antioxidant activity
Oxidative stress has long been implicated in a wide range of disorders, including atherosclerosis, hypertension, cardiovascular disease, diabetes, cancer, and neurological diseases [6,7]. Results of several studies have shown that Cistus species is higher in phenolic compounds with substantial antioxidant capabilities. Several assays were used to identify this activity such as DPPH, FRAP, ATBS and others.
According to the research by Andrade and colleagues in 2009, CL has a substantial antioxidant impact with an EC50 value of 7.85g/ mL. A further study showed that CL extracts were able to effectively scavenge DPPH radicals at concentrations of 1000g/ml. CL Extracts were found to have antioxidant ability comparable to that of trolox and ascorbic acid [27]. Free radical scavenging properties of different extracts from different CL parts and essential oils of CL, were identified, with methanolic extract obtained from the leaf extract that was found to be more effective than the other parts of the plant [97.8%], which was similar to that of ascorbic acid [30]. Moreover, extracts of CL from Poland exhibited a significant antioxidant activity with IC50 ranging from 4.08 to 10.20μg/ml [13]. Enzymatic hydrolysates from pollen of CL showed also a significant antioxidant activity [54], at the concentration of 10mg/ml, Cistus extract was able to scavenge 50% of the DPPH radical, with papain hydrolysate showing the best results [75%]. Extracts of CL Hexanic, dichloromethane, methanolic and aqueous exhibited an interesting antioxidant activity, at a high dose [2mg/ml], the effect of the four extracts was equivalent to that obtained with BHT, used as reference molecule [55]. At lesser doses, the methanolic extract exhibited a significantly high antioxidant activity, greater than that obtained with BHT [56]. Further evidence of antioxidant properties of CL was found in another investigation, wherein extracts showed significant radical-scavenging activity, with an IC50 value of between 3.0 and 4.7g/mL [56]. It was shown that the methanolic extract of CL had significant radical scavenging activity when reacted with ABTS + radicals [27]. Also, FRAP assays were used to determine the antioxidant power of CL. In fact, extracts of branches and stems of this plant had strong ferric ion reducing activities that ranged from 6.6 to 10.1mM Fe2+/g extract [56]. At the concentration of 250μg/ml, methanolic and ethanolic extracts chelated ferrous ions by 7.11 % and 6.04 %, respectively [27]. However, at 5,000μg/ml, the methanolic extract chelated ferrous ions by 73.15 %, which was greatest. Using the FRAP assay, that the aqueous extract of CL had antioxidant activity [117.72 4.38mmol Fe2+/100g dw] [56]. The buffered egg yolk lipid peroxidation assay also showed that the methanolic extract of this plant worked better as an antioxidant [EC50 value of 0.5mg/ml] [27]. Using the lipid peroxidation assay, it was found that the abilities of pepsin and trypsin hydrolysates from CL that were higher than those of 1mM a-tocopherol and ascorbic acid [57]. The ethanolic extract of CL from Morocco exhibited a significant antioxidant effect recording an IC50 value of 266.6 ± 0.828μg/mL with DPPH assay, and a high reducing power 0.494 ± 0.035 using the FRAP test [14].
In extracts of plants, the presence of phenolic chemicals is often associated with large amounts of antioxidant activity. CL was found to be rich in phenolic compounds and therefore they can be responsible of this interesting activity reported in several studies. In fact, Cistus ingredients derived from leaf extracts and essential oils are included in some cosmetic products as antioxidants in their formulations [5]. Based on results of all these studies, this activity reveals interesting applications for CL extracts as an agent preventing free radicals, which leads to the oxidation of lipids in food and biological systems (Table 2).
Table 2:Antioxidant activity of C. ladanifer.
Cytotoxic activity
Chemotherapy and radiotherapy have long been used to treat cancer. These treatments target cells that divide quickly but have a number of side effects on normal cells [3,58,59]. Today, new therapeutic approaches are being developed and researchers have been interested in the use of CL and its compounds to specifically target tumor cells without affecting normal cells. Few reports are describing the anti -proliferative potent of this plant.
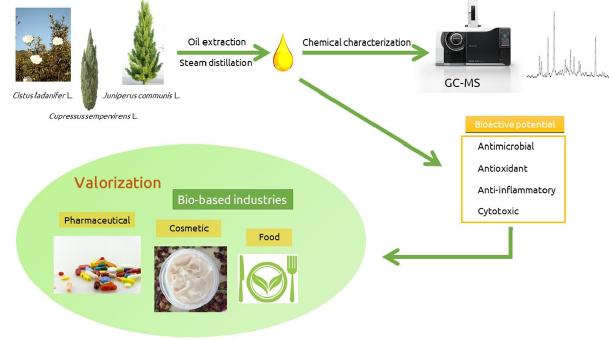
According to Xavier et al, C. ladanifer EOs exhibited the highest potential on NCI-H4100 cell lines. Nevertheless, some EOs revealed toxicity against non-tumoral cells but generally presented a GI50 value higher than that of the tumor cell lines (Figure 8) (Xavier et al., 2021).
Figure 8:Representation of the biological activities of C. Ladanifer according to Xavier.
The aqueous extract of CL leaves from Spain was studied for its cytotoxic activity on cell lines of pancreas, breast and colon [7]. In this investigation, the most sensitive cells were the pancreatic cell line M220, breast cancer cells MCF7/HER2 and JIMT-1 cells, with CC50 ranging from 0.49mg/ml to 1.6-2mg/ml. This activity was associated with the presence of punicalagins and Ellagitannins that are already known for their anticancer potencies [100-62]. Two human skin cancer cells, malignant melanoma [A375] and squamous cell carcinoma, [SCC-15] were used to illustrate effects of CL on skin cancer [13]. Extracts of CL also exhibited promising potential against the two cell lines. In the same study, the cytotoxic activity of CL extracts was correlated with the tyrosinase inhibitory activity. Using the MTT assay it was demonstrated that extracts of CL decreased cells viability [12]. Another study revealed that extracts of CL from Morocco possess an important antiproliferative activity against breast [MDA-MB-231], prostate [22Rv1] and hepatocarcinoma [HepG2] cancerous cell lines, the Hexanic extract presented the highest antiproliferative activity against 22Rv1 and MDA-MB-231 recording IC50 values 11.32 ± 2.12μg/mL and 82.4 ± 1.12μg/mL, respectively while the dichloromethane extract was revealed to be more effective against HepG2 with an IC50 of 31.54 ± 0.242μg/mL [14] (Table 3).
Table 3:Antioxidant activity of C. ladanifer.
Anti-inflammatory and anti-nociceptive activities
Analgesic and anti-inflammatory drugs are used to treat inflammatory illnesses. NSAIDs are commonly utilized, however long-term usage can cause various negative effects. The aqueous extract of CL has been shown to have significant anti-inflammatory activity. In fact, the aqueous extract of this plant reduced inflammation and stopped edema by 66.67 to 77.78 % more than the anti-inflammatory drug indomethacin [66.67%]. In fact, pretreatment with an aqueous extract of the CL at doses of 150, 175, and 200mg/kg b.m. reduced edema paw inflammation weight by 66.67%, 67.65 and 77.78%, respectively. This was more effective than the reference drug indomethacin [66.67%], which was used as a positive control [63]. Analgesic properties in a heat-induced pain paradigm were also discovered in this investigation. Analgesic effects of CL were shown to be more effective than Tramadol at dosages of 150, 175, and 200mg/kg b.w. [64]. Furthermore, these anti-inflammatory and anti-nociceptive effects were linked to the impact of CL inflammatory mediators, including histamine, serotonin, and proinflammatory cytokines. The presence of flavonoids such as kaempferol and its derivatives may be a factor in this action of CL. Flavonoids have been shown to serve as possible inhibitors of the enzymes COX, lipoxygenase, and nitric oxide synthase [65,66].
Hypoglycemic and hypolipidemic activities
In terms of mortality and morbidity, diabetes has always been a problem. Due to some limitations of synthetic drugs, the hunt for novel medicines in the treatment of diabetes continues. CL has been shown to have anti-diabetic properties. Glibenclamide’s antidiabetic impact was equivalent to that of CL extract in diabetic rats, according to the results of this study. In fact, diabetic rats that received 500mg/kg, b.m. of CL extract had significantly lower blood glucose levels than those of control rats. When CL extract was taken orally on a regular basis, the FBG [fasting blood glucose level] decreased significantly compared to diabetes control. In addition, CL reduced the increase in blood glucose levels significantly compared to management of diabetes. A 12.5% decrease in glucose levels was seen in diabetic rats treated with CL extract, compared to diabetic control rats [9]. Anti-diabetic properties of CL bee pollen have also been investigated [67]. In fact, the bee pollen extract at various dosages of 5,10,20mg/100g body weight was shown to avoid numerous abnormalities in diabetic rats.
Blood tests showed that the hypolipidemic impact reduced total cholesterol, triglycerides, and low-density lipoprotein cholesterol significantly. Diabetic patients are compared to diabetic controls. Glibenclamide [500mg/kg, b.m.] was also shown to reduce the levels of alanine aminotransferase and aspartate aminotransferase in diabetic rats fed with CL extract [500mg/kg b.m.]. Treatment with CL extract for 28 days resulted in a substantial decrease in urea and creatinine levels in the diabetic group as compared to the diabetic group used as control [9]. Significant anti-diabetic activity observed in CL may be due to the synergistic effect of different classes of bioactive compounds present in the plant extract, primarily flavonoids are capable of stimulating glucose uptake in peripheral tissues and insulin secretion by modifying the concentration of Ca2+, thereby reducing oxidative stress in diabetic subjects [68].
Anticancer activity: Liver, prostate and breast cancer
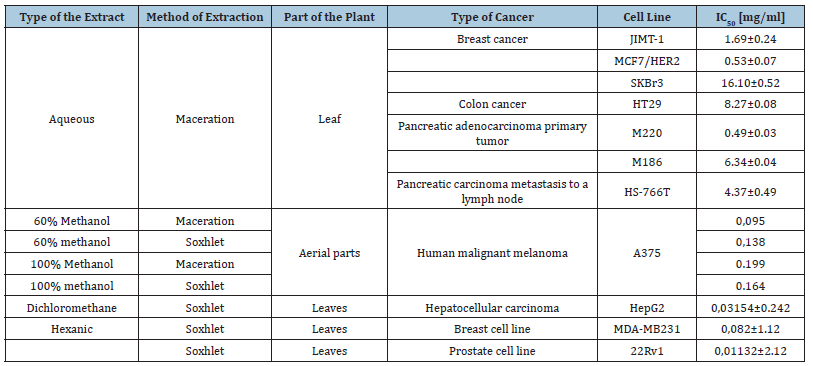
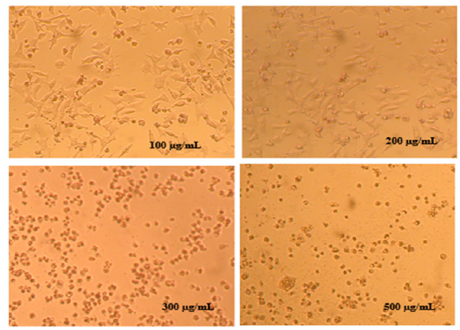
The research team of CNESTEN has explored in 2022 the chemical composition and evaluated the antiproliferative properties of C. ladanifer against three human cancer cell lines: liver, prostate, and breast. To our knowledge, the antiproliferative effects of C. ladanifer on liver (HepG2), prostate (22Rv1), and breast (MDA-MB-231) cancer cells have not been investigated before. Our findings demonstrate strong antioxidant effects and notable antiprostate cancer activity, with an IC50 value as low as 11.32±2.126μg/ mL (Bouothmany et al., 2022) (Figure 9).
Figure 9:Cytopathic effects in MDA-MB-231 cancer cells after 72h post-treatment with increasing concentration of Cistus ladanifer hexanic extract (Bouothmany et al., 2022).
Wound healing
The herbal Cistus ladanifer preparations showed potential for anti-inflammatory and skin repair. Also, it exhibited a promising biocompatible profile on both cell lines, demonstrating antiinflammatory properties. For its application in folk cosmetics and hygiene products. Its use in acne vulgaris has also been reported. C. ladanifer is traditionally used in remedies for wounds, ulcers, and other skin ailments such as psoriasis and eczema. Its application has been found useful due to its anti-inflammatory, astringent, wound healing, and antiseptic properties (Oliveira et al., 2023).
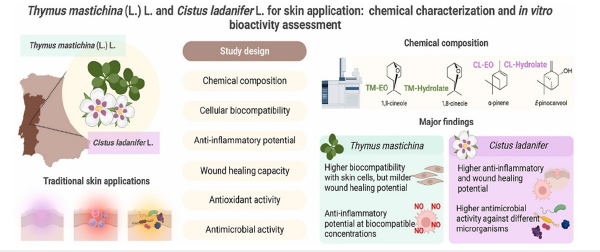
Additionally, their essential oils displayed antimicrobial activity against various microorganisms in vitro. These observed bioactivities corroborate some traditional skin uses, particularly in terms of antiseptic, wound healing, and anti-inflammatory applications (Oliveira et al., 2023) (Figure 10).
Figure 10:Schematic representation of the skin application of Cistus ladanifer: from chemical characterization to in-vitro assays according to (Oliveira et al., 2023).
Diuretic activity
Diuretics are often prescribed for conditions defined by an excess of extracellular fluid, such as chronic renal disease, nephrotic syndrome, cirrhosis, and heart failure. Numerous therapeutic plants have been discovered to have diuretic properties. It has been shown that a single dosage of CL or daily doses over a period of 8 days had a strong diuretic effect. Compared to the control group, the daily administration of an aqueous extract of CL significantly increased the excretion of creatinine, sodium, and potassium. A substantial increase in creatinine clearance was found in groups that had been administered CL. In addition, the extract induced a little decrease in urine osmolarity [69]. This plant has the ability to enhance salt, potassium, and creatinine excretion via the urine without producing hypokalemia or hyponatremia. thiazide diuretics [9,69] and so may be used as a future diuretic without hypokalemia, which is a typical finding with the use of loop diuretics or hydro-chlorothiazides. Flavonoids have been shown to have diuretic properties [70]. These studies relate the observed activity to the flovoinds contained in CL extract, which include isoflavonoids such as genistein and daidzein, which inhibit the Na+-K+ -2Cl cotransporter and induce natriuresis and kaluresis [71,72].
Conclusion
The stone rose, Cistus ladaniferus is a valuable resource that deserves further attention. This plant possesses various pharmacological properties resulting from the presence of compounds, including phenolic and terpenes compounds with biological activities. This draws attention to the possibility of using Cistus extracts and bioactive substances in the treatment of different diseases.
References
- Petrovska B (2012) Historical review of medicinal plants′ usage. Pharmacogn Rev 6(11): 1-5.
- Sharma SB, Gupta R (2015) Drug development from natural resource: A Systematic Approach. MRMC 15(1): 52-57.
- Bouyahya A, Abrini J, Et-Touys A, Bakri Y, Dakka N (2017) Indigenous knowledge of the use of medicinal plants in the North-West of Morocco and their biological activities. European Journal of Integrative Medicine 13: 9-25.
- Rehman FUr, Maria K, Muhammad A, Bahman FN, Nageen N, et al. (2021) Importance of medicinal plants in human and plant pathology: A review. Int J Phar & Biomedi Rese 8(2): 1-11.
- Barrajón CE, Tomás ML, Morales SA, Bruñá NM, López DS, et al. (2016) Chapter 74 rockroses [Cistus sp.] oils. Essential Oils in Food Preservation, Flavor and Safety. Elsevier, pp. 649-658.
- Stępień A, Aebisher D, Bartusik AD (2018) Biological properties of cistus species. Eur J Clin Exp Med 16(2): 127-132.
- Barrajón C, Fernández AS, Saura D, Guillén E, Fernández GA, et al. (2010) Cistaceae aqueous extracts containing ellagitannins show antioxidant and antimicrobial capacity, and cytotoxic activity against human cancer cells. Food and Chemical Toxicology 48(8-9): 2273-2282.
- Vitali F, Pennisi G, Attaguile G, Savoca F, Tita B (2011) Antiproliferative and cytotoxic activity of extracts from Cistus in canus L. and Cistus monspeliensis L. on human prostate cell lines. Natural Product Research 25(3): 188-202.
- El-Kabbaoui M, Chda A, Azdad O, Mejrhit N, Aarab L, et al. (2016) Evaluation of hypoglycemic and hypolipidemic activities of aqueous extract of Cistus ladaniferus in streptozotocin-induced diabetic rats. Asian Pacific Journal of Tropical Biomedicine 6(12): 1044-1049.
- Barros L, Dueñas M, Alves CT, Silva S, Henriques M, et al. (2013) Antifungal activity and detailed chemical characterization of Cistus ladanifer phenolic extracts. Industrial Crops and Products 41: 41-45.
- El-Karkouri J, Bouhrim M, Al Kamaly OM, Mechchate H, Kchibale A, et al. (2021) Chemical composition, antibacterial and antifungal activity of the essential oil from Cistus ladanifer L. Plants 10(10): 2068.
- Andrade D, Gil C, Breitenfeld L, Domingues F, Duarte AP (2009) Bioactive extracts from Cistus ladanifer and Arbutus unedo L. Industrial Crops and Products 30(1): 165-167.
- Gaweł BK, Kukula KW, Hoian U, Czop M (2020) Characterization of cistus × incanus l. and cistus ladanifer l. extracts as potential multifunctional antioxidant ingredients for skin protecting cosmetics. Antioxidants 9(3): 202.
- Alves FJ, Duarte LC, Fernandes MC, Pereira H, Carvalheiro F (2023) Cistus ladanifer as a potential feedstock for biorefineries: A Review. Energies 16(1).
- Papaefthimiou D, Papanikolaou A, Falara V, Givanoudi S, Kostas S, et al. (2014) Genus cistus: A model for exploring labdane-type diterpenes’ biosynthesis and a natural source of high value products with biological, aromatic, and pharmacological properties. Front Chem 2: 35.
- Barrajón CE, Fernández AS, Roldán C, Guillén E, Saura D, et al. (2011) A systematic study of the polyphenolic composition of aqueous extracts deriving from several Cistus genus species: Evolutionary relationship. Phytochem Anal 22(4): 303-312.
- Gülz PG, Herrmann T, Hangst K (1996) Leaf trichomes in the genus Cistus. Flora 191(1): 85-104.
- Du Plessis SP, Rink A, Goodall V, Kaplan H, Jubase N, et al. (2018) Assessment and management of the invasive shrub, Cistus ladanifer, in South Africa. South African Journal of Botany 117: 85-94.
- Alves FJ, Miranda I, Duarte LC, Roseiro LB, Lourenço A, et al. (2020) Cistus ladanifer as a source of chemicals: Structural and chemical characterization. Biomass Conv Bioref 10(2): 325-337.
- Mahmoudi H, Aouadhi C, Kaddour R, Gruber M, Zargouni H, et al. (2016) Comparison of antioxidant and antimicrobial activities of two cultivated Cistus species from Tunisia. Biosci J 32(1): 226-237.
- Raimundo JR, Frazão DF, Domingues JL, Quintela SC, Dentinho TP, et al. (2018) Neglected mediterranean plant species are valuable resources: The example of Cistus ladanifer. Planta 248(6): 1351-1364.
- Gomes PB, Mata VG, Rodrigues AE (2005) Characterization of the Portuguese-grown cistus ladanifer essential oil. Journal of Essential Oil Research 17(2): 160-165.
- Santos SE, Balseiro RM, Abreu MM, Macías F (2017) Bioextracts of Cistus ladanifer L. growing in são domingos mine as source of valuable compounds. Journal of Geochemical Exploration 174: 84-90.
- Barrero AF, Alvarez MEJ, Chahboun R, Arteaga AF (2004) Degradation of the side chain of [−]‐sclareol: A very short synthesis of nor ‐ambreinolide and ambrox. Synthetic Communications 34(19): 3631-3643.
- Brenna E, Fuganti C, Serra S (2003) Enantioselective perception of chiral odorants. Tetrahedron: Asymmetry 14[6]: 1-42.
- Amensour M, Sendra E, Pérez AJA, Skali SN, Abrini J, et al. (2010) Antioxidant activity and chemical content of methanol and ethanol extracts from leaves of rockrose [Cistus ladaniferus]. Plant Foods Hum Nutr 65(2): 170-178.
- Ramalho PS, De Freitas VAP, Macedo A, Silva G, Silva AMS (1999) Volatile components of Cistus ladanifer Flavour Fragr J 14(5): 300-302.
- Loizzo MR, Ben JM, Senatore F, Bruno M, Menichini F, et al. (2013) Chemistry and functional properties in prevention of neurodegenerative disorders of five Cistus species essential oils. Food and Chemical Toxicology 59: 586-594.
- Zidane H, Elmiz M, Aouinti F, Tahani A, Wathelet J (2013) Chemical composition and antioxidant activity of essential oil, various organic extracts of Cistus ladanifer and Cistus libanotis growing in Eastern Morocco. Afr J Biotechnol 12(34): 5314-5320.
- Jerónimo E, Alfaia CMM, Alves SP, Dentinho MTP, Prates JAM, et al. (2012) Effect of dietary grape seed extract and Cistus ladanifer L. in combination with vegetable oil supplementation on lamb meat quality. Meat Science 92(4): 841-847.
- Guimarães R, Barros L, Carvalho AM, Sousa MJ, Morais JS, et al. (2009) Aromatic plants as a source of important phytochemicals: Vitamins, sugars and fatty acids in Cistus ladanifer, Cupressuslusitanica and Eucalyptus gunnii leaves. Industrial Crops and Products 30(3): 427-430.
- Zalegh I, Akssira M, Bourhia M, Mellouki F, Rhallabi N, et al. (2021) A review on Cistus sp.: Phytochemical and antimicrobial activities. Plants 10(6): 1214.
- Vogt T, Gerhard GP (1994) Accumulation of flavonoids during leaf development in Cistus laurifolius. Phytochemistry 36(3): 591-597.
- Chaves LN, Ferrer De La CI, Alías GJ (2019) Autotoxicity of diterpenes present in leaves of Cistus ladanifer L. Plants 8(2): 27.
- Dentinho MTP, Moreira OC, Pereira MS, Bessa RJB (2007) The use of a tannin crude extract from Cistus ladanifer L. to protect soya-bean protein from degradation in the rumen. Animal 1(5): 645-650.
- Sosa T, Chaves N, Alias JC, Escudero JC, Henao F, et al. (2004) Inhibition of mouth skeletal muscle relaxation by flavonoids of Cistus ladanifer l.: A plant defense mechanism against herbivores. J Chem Ecol 30(6): 1087-1091.
- Alías JC, Sosa T, Valares C, Escudero JC, Chaves N (2012) Seasonal variation of Cistus ladanifer l. Diterpenes. Plants 1(1): 6-15.
- Fernández AS, Barrajón CE, Micol V, Segura CA, Fernández GA (2010) High-performance liquid chromatography with diode array detection coupled to electrospray time-of-flight and ion-trap tandem mass spectrometry to identify phenolic compounds from a Cistus ladanifer aqueous extract: Identification of phenolic compounds from Cistus ladanifer. Phytochem Anal 21(4): 307-313.
- Boukili M, Chakir S, Rhazi FF, Fikri BK, Halaoui Z, et al. (2018) Chemical composition and antimicrobial activity of the essential oil of cistus ladanifer var. Maculatus dun. JMBFS 8(3): 925-930.
- Frazão DF, Raimundo JR, Domingues JL, Quintela SC, Gonçalves JC, et al. (2018) Cistus ladanifer (Cistaceae): A natural resource in Mediterranean-type ecosystems. Planta 247(2): 289-300.
- Oller LJL, Rodríguez R, Cuerva JM, Oltra JE, Bazdi B, et al. (2005) Composition of the essential oils of Cistus ladaniferus and monspeliensis from Morocco. Journal of Essential Oil Research 17(5): 553-555.
- Teixeira S, Mendes A, Alves A, Santos L (2007) Simultaneous distillation-extraction of high-value volatile compounds from Cistus ladanifer L. Analytica Chimica Acta 584(2): 439-446.
- Tomás ML, Morales SA, Barrajón CE, Roldán SC, Segura CA, et al. (2013) Correlation between the antibacterial activity and the composition of extracts derived from various Spanish Cistus species. Food and Chemical Toxicology 55: 313-322.
- Greche H, Mrabet N, Zrira S, Ismaïli AM, Benjilali B, et al. (2009) The volatiles of the leaf oil of Cistus ladanifer L. var. albiflorus and labdanum extracts of Moroccan origin and their antimicrobial activities. Journal of Essential Oil Research 21(2): 166-173.
- Verdeguer M, Blázquez MA, Boira H (2012) Chemical composition and herbicidal activity of the essential oil from a Cistus ladanifer L. population from Spain. Natural Product Research 26(17): 1602-1609.
- Bechlaghem K, Allali H, Benmehdi H, Aissaoui N, Flamini G (2019) Chemical analysis of the essential oils of three cistus species growing in North West of Algeria. Algeria Agric Conspec Sci, pp. 283-293.
- Rossi PG, Berti L, Panighi J, Luciani A, Maury J, et al. (2007) Antibacterial action of essential oils from Corsica. Journal of Essential Oil Research 19(2): 176-182.
- Guinoiseau E, Lorenzi V, Luciani A, Tomi F, Casanova J, et al. (2011) Susceptibility of the multi-drug resistant strain of Enterobacter aerogenes EA289 to the terpene alcohols from Cistus ladaniferus essential oil. Nat Prod Commun 6(8): 1159-1162.
- Vieira M, Bessa LJ, Martins MR, Arantes S, Teixeira APS, Mendes Â, et al. (2017) Chemical composition, antibacterial, antibiofilm and synergistic properties of essential oils from eucalyptus globulus Labill. and seven mediterranean aromatic plants. Chem Biodiversity 14(6): e1700006.
- Mrabet N, Lahlou H, Benjilali B (1999) Effect of Moroccan Cistus ladaniferus L. [rockrose] extracts on the growth of four fungi. Cryptogamie Mycologie 20(1): 23-33.
- Benayad N, Mennane Z, Charof R, Hakiki A, Mosaddak M (2013) Antibacterial activity of essential oil and some extracts of Cistus ladaniferus from Oulmes in Morocco. J Mater Environ Sci 4(6): 1066-1071.
- Lekbach Y, Xu D, El Abed S, Dong Y, Liu D, et al. (2018) Mitigation of microbiologically influenced corrosion of 304L stainless steel in the presence of Pseudomonas aeruginosa by Cistus ladanifer leaves extract. International Biodeterioration & Biodegradation 133: 159-169.
- Köse MD, Tekin BN, Bayraktar O, Duman ET, Başpınar Y (2017) Antioxidant and antimicrobial properties of cistus ladanifer. International Journal of Secondary Metabolite 4(3): 434-44.
- Bakrim H, Zerrouk MH, El Galiou O, Attaleb M, Benbacer L, et al. (2021) Bioactive properties of natural compounds extracted from leaves of cistus ladanifer. Eco Env & Cons 1563-1574.
- Barrajón CE, Fernández AS, Saura D, Guillén E, Fernández GA, et al. (2010) Cistaceae aqueous extracts containing ellagitannins show antioxidant and antimicrobial capacity, and cytotoxic activity against human cancer cells. Food and Chemical Toxicology 48(8-9): 2273-2282.
- Alves FJ, Duarte LC, Fernandes MC, Pereira H, Carvalheiro F (2019) Hydrothermal treatments of Cistus ladanifer industrial residues obtained from essential oil distilleries. Waste Biomass Valor 10(5): 1303-1310.
- Tripathi P, Singh A (2017) Natural resources from plants in the treatment of cancer: An update. Asian J Pharm Clin Res 10(7): 13.
- Seeram N, Lee R, Hardy M, Heber D (2005) Rapid large scale purification of ellagitannins from pomegranate husk, a by-product of the commercial juice industry. Separation and Purification Technology 41(1): 49-55.
- Heber D (2008) Multitargeted therapy of cancer by ellagitannins. Cancer Letters 269(2): 262-268.
- Syed D, Afaq F, Mukhtar H (2007) Pomegranate derived products for cancer chemoprevention. Seminars in Cancer Biology 17(5): 377-385.
- El Hamsas El YA, El Mansouri L, Boukhira S, Daoudi A, Bousta D (2016) In Vivo anti-inflammatory and analgesic effects of aqueous extract of Cistus ladanifer l. from morocco. American Journal of Therapeutics 23(6): e1554-e1559.
- Sayah K, Marmouzi I, Naceiri MH, Cherrah Y, Faouzi MEA (2017) Antioxidant activity and inhibitory potential of Cistus salviifolius [L.] and Cistus monspeliensis [L.] aerial parts extracts against key enzymes linked to hyperglycemia. Biomed Research International 2017: 2789482.
- Tahiri O, Atmani KD, Sanchez FS, Aparicio SM, Alarcón-de-la-LC, et al. (2017) The flavonol-enriched Cistus albidus chloroform extract possesses in vivo anti-inflammatory and anti-nociceptive activity. Journal of Ethnopharmacology 209: 210-218.
- Rauter AP, Martins A, Borges C, Mota FH, Pinto R, et al. (2010) Antihyperglycaemic and protective effects of flavonoids on streptozotocin-induced diabetic rats. Phytotherapy Research 24(Suppl 2): 133-138.
- El Menyiy N, Al-Waili N, El-Haskoury R, Bakour M, Zizi S, et al. (2018) Potential effect of Silybummarianum L. and Cistus ladaniferus L. extracts on urine volume, creatinine clearance and renal function. Asian Pacific Journal of Tropical Medicine 11(6): 393.
- Zeng X, Xi Y, Jiang W (2018) Protective roles of flavonoids and flavonoid-rich plant extracts against urolithiasis: A review. Cri Rev Food Sci Nutr 59(13): 2125-2135.
- Martínez RM, Lou JM, Mayoral JA, Alda JO, Giménez I [2018] Soy isoflavonoids exhibit in vitro biological activities of loop diuretics. The American Journal of Clinical Nutrition 68(6): 1354S-1357S.
- Karim H, Boubaker H, Askarne L, Cherifi K, Lakhtar H, et al. (2017) Use of Cistus aqueous extracts as botanical fungicides in the control of Citrus sour rot. Microbial Pathogenesis 104: 263-267.
- Thielmann J, Muranyi P, Kazman P (2019) Screening essential oils for their antimicrobial activities against the foodborne pathogenic bacteria Escherichia coli and Staphylococcus aureus. Heliyon 5(6): e01860.
- Benali T, Bouyahya A, Habbadi K, Zengin G, Khabbach A, et al. (2020) Chemical composition and antibacterial activity of the essential oil and extracts of Cistus ladaniferus subsp. ladanifer and Mentha suaveolens against phytopathogenic bacteria and their ecofriendly management of phytopathogenic bacteria. Biocatalysis and Agricultural Biotechnology 28: 101696.
- Nagai T, Inoue R, Inoue H, Suzuki N (2002) Scavenging capacities of pollen extracts from cistus ladaniferus on autoxidation, superoxide radicals, hydroxyl radicals, and DPPH radicals. Nutrition Research 22(4): 519-526.
- Lichota A, Gwozdzinski K (2018) Anticancer activity of natural compounds from plant and marine environment. IJMS 19(11): 3533.
© 2024 Boutaina Addoum. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






