- Submissions

Full Text
Journal of Biotechnology & Bioresearch
Cuttings Vegetative Multiplication of Smooth Caper (Capparis Spinosa Inermis)
Benaoun Hanine1*, ZouaouiRefka2, Elaloui Meriem2, Bahri Salima2, Abassi Mejda2 and Ammari Youssef2
1Faculty of Sciences of Bizerte, University of Carthage, Jarzouna 7021, Tunisia
2National Institute of Research in Rural Engineering, Water and Forests (INRGREF), University of Carthage, Tunisia
*Corresponding author:Benaoun Hanine, Faculty of Sciences of Bizerte, University of Carthage, Jarzouna 7021, Tunisia
Submission: January 20, 2023;Published: April 20, 2023

Volume4 Issue4April , 2023
Abstract
The present study consists in knowing the techniques of asexual multiplication (woody and herbaceous cuttings) of caper (Capparis Spinosa Inermis) family of the capparidaceae, species with high socio-economic value. Cuttings were incised at the base of their ends and then treated with growth hormones at different concentrations(1000, 2000, 5000ppm AIB), grown on 4 types of substrate (Substrate1 (1/3perlite+1/3 sand+1/3 peat), Substrate 2 (1/4 sand+3/4 peat), Substrate 3 (1/2 sand+1/2 peat), Substrate4 (3/4 sand +1/4 peat). Other cuttings were planted in the absence of hormone, according to their order of branching in relation to the mother plants. The main results showed that there are significant differences (p<0.05) between the two types of cuttings, type of substrate and hormone treatment. In fact woody cuttings of smooth caper gave the best recovery percentages (>80%) in substrate 1 with 2000ppm AIB. It should also be noted that herbaceous cuttings are very sensitive to the chemicals presented by the growth hormone. Indeed, the best recovery percentage was obtained for the untreated ones (66%). Since the number of flower buds, which represents the production of capers, is very limited for both cutting techniques.
Keywords:Smooth caper; Asexual multiplication; Woody cuttings; Herbaceous cuttings; substrate; Branching order
Introduction
The smooth caper (Capparis Spinosa Inermis) is a species with high socioeconomic value [1,2]. It is a spontaneous, xerophytic, domesticated shrub of the capparidaceae family [3,4] with multiple uses (food, medicinal, cosmetic, ornamental…) [5-7]. Its area of distribution is spread out on the Mediterranean rim [1,4,8], able to grow wild on dry and poor soils of arid and semi-arid regions in rocky, marly places and on the cracks of old walls [9]. The caper can play a very useful ecological role in regions with a dry and hot climate for protection against erosion [10]. In particular, the main product of this species is the caper, it is a flagship product very appreciated and requested by the local and international market [11].The economic importance of capers led to a significant increase in both the area cultivated and production levels in the late 1980s [12]. The beneficial effect of capers as a condiment plant has been confirmed by food chemistry studies [13,14]. The therapeutic effectiveness of the plant organs, inspired by ethnobotanical references, seems to give results for natural anti-cancer and anti-inflammatory treatments. Many phytopharmaceutical scientists are currently actively researching the plant’s molecule. The world production of capers is estimated between 15 and 20000 tons/year [15]. In 2005, the main caper exporting countries were: Morocco, Spain and France. It should be noted that Morocco is the first caper producing and exporting country in the world with an annual production of 20,000 tons and an export of 15-20,000 tons [16]. The national production of capers remains very limited compared to the production of other countries around the Mediterranean. In fact, it has fluctuated between 160T and 200T per year coming essentially from the wild water table [16]. Under natural conditions, the caper multiplies by way of its seeds consumed on the fruits burst by birds. Indeed, the seeds sufficiently well digested through the intestinal transit and rejected in the droppings of birds (partridge, pigeons, etc..), will germinate in small seedlings that will take several months to grow and reach a significant size [17]. The research works in Tunisia and in other Mediterranean countries show that the modes of multiplication and production of quality plants are the main obstacles which slow down the extension of the culture of this shrub [6,18]. The traditional mode of multiplication essentially by sowing gives a low percentage of germination and heterogeneity as well in terms of productivity as in terms of quality of capers [18,19]. This species encounters different types of problems mainly those related to its propagation where the success rate of cuttings is low [20,21]. The objective of this study is therefore to develop and know the techniques of vegetative propagation of this species which still poses difficulties in order to use it to develop innovative and productive agro-systems.
Method and Materials
Plant material
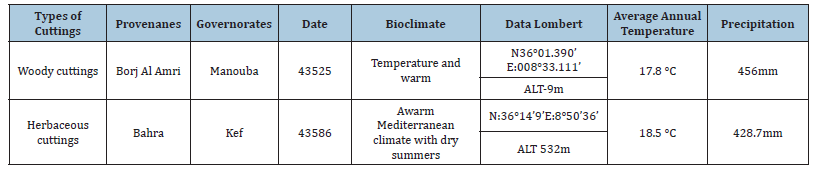
The plant material used is in the form of woody cuttings that were taken in March 2019 from adult caper mother plants grown in the Borjtoumi area (Manouba Governorate, Tunisia). The herbaceous cuttings were taken from wild vigorous mother plants in the region of Bahra (North-West of Tunisia, governorate Kef) in May 2019. The new tender shoots are with heel, that is to say they include a fragment of wood of the previous year and a green part of the year (Table 1).
Table 1:Geographic data of the provenances of collection of the smooth caper cuttings.
Methods
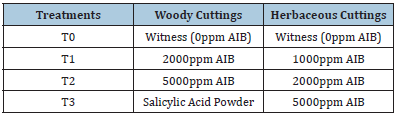
Woody cuttings were cut into 15-20cm long pieces and transplanted into containers filled with four types of sand, peat and perlite substrates of different proportions: Substrate 1 (1/3 perlite + 1/3 sand +1/3 peat), Substrate 2 (1/4 sand+3/4 peat), Substrate 3 (1/2 sand+1/2 peat) and Substrate 4 (3/4 sand+1/4 peat). The containers were placed in a semi-controlled greenhouse at INRGREF (Ariana Governorate). Each container contained 20 cuttings with 3 replicates per substrate. Four different treatments were applied to each substrate (Table 2). The herbaceous cuttings were subjected to four different treatments with the growth hormone, auxin (Table 2). The same device as for woody cuttings was applied to herbaceous cuttings at INRGREF in May 2019. For the cuttings chosen according to the order of branching, the experimental device was placed in the forest nursery of Borj El Amri (Manouba governorate), with a temperate and warm bioclimatic. The propagation by woody cuttings of the unarmed caper plant was divided into 3 lots with different order of branching (branching of order 1, order 2 and order 3) on a substrate based on sand.
Table 2:Details of hormonal treatments applied to the cuttings of the smooth caper.
Settings
A. Percentage of recovery of cuttings
B. Monitoring of morphological parameters (number of
vegetative buds, number of leaves per bud, length of vegetative
shoots, diameter of vegetative shoots, number of flower buds
...) for each week.
C. Root length and root number will be determined at the
end of the experiment.
D. Mortality rate
Statistical analysis
For data interpretation, the results were analyzed by IBMSPSS software, Excel Stat 2015 using analysis of variance (ANOVA), Duncan and correlation between different morphological parameters.
Results
Propagation of the smooth caper by woody cuttings Effect of growth hormone on woody cuttings
The analysis of the data obtained from the propagation of the smooth caper (Capparis Spinosa Inermis) by woody cuttings showed a significant difference (p<0.05) under the effect of substrate and applied treatment (Table 3). In fact, the recovery rate of new shoots showed the highest value (83.33%) for substrate 1 (1/3 perlite+1/3 sand+1/3 peat) with hormonal treatment T1 (2000ppm) while the lowest value is recorded with treatment T3 (Salicylic acid in powder) for all types of substrate. In addition, treatment T1 induced the highest number of buds (6 buds), number of roots (9 roots) and diameter of new shoots (1.86mm). For T3 treatment, it showed the highest mortality rate with 46.66%. Regarding the length of new shoots, it presented the highest value (12.33cm) in substrate 1 for untreated cuttings. Thus, there was a very limited caper production in our preliminary asexual propagation trial (13 capers) for the four types of substrates. However, the results for root length, the best value (13.3cm) was obtained in substrate 2 (1/4 sand+3/4 peat) for cuttings treated with 5000ppm AIB(T2). While the results of the number of leaves per bud showed that the most suitable substrates are S2 (1/4 sand+3/4peat) and S3 (1/2 sand+1/2peat) for the T2 treatment (5000ppm) with 16 and 17 leaves respectively.
Table 3:Variation in recovery rate and morphological characters of woody cuttings: Number of buds (NB); Number of leaves/bud(NF/B); Number of floral buds(NBF), Length of new shoots(L), Number of roots(NR), Length of roots(LR)and diameter(D), studied in 4 types of substrate(S1 (1/3 perlite + 1/3 sand +1/3peat), S2 (1/4 sand + 3/4peat), S3 (1/2 sand+ 1/2peat) and S4 (3/4 sand+ 1/4 peat) and 4 types of treatment(T0: Control; T1: 2000ppm AIB; T2: 5000ppm AIB and T3: Salicylic acid).
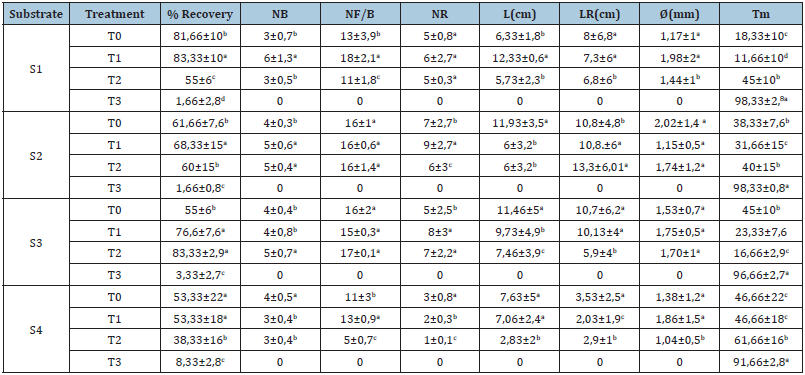
Note: For each parameter (row, column), the means followed by the same letter are not significantly different at the 5% threshold according to the Turkey test (n=5 ± standard deviation).
Correlation between different morphological parameters of woody cuttings
Table 4 summarizes the correlation between different morphological parameters of woody cuttings of smooth caper. It should be noted that there is a strong positive correlation, highly significant (p<0.001) recorded between the number of buds, number of leaves/stem, number of flower buds, length of new shoots, number of roots, length of roots and diameter.
Table 4:Pearson correlation between different morphological parameters of woody cuttings: Number of buds (NB), Number of leaves/stem (NF/B), Number of floral buds(NBF), Length of new shoots(L), Number of roots(NR), Length of roots(LR)and diameter(Ø),(n=5).
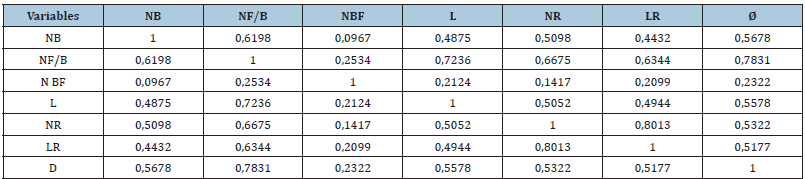
Note: For each parameter (row, column), the means followed by the same letter are not significantly different at the 5% threshold according to the Tukey test (n=5±standard deviation).
Effect of twig order on the budding of smooth caper
In testing the branching order of woody cuttings of the smooth caper in relation to the mother plant, the results obtained showed that there was no effect of branching order (p>0.05) on the majority of morphological parameters. Only, there is a significant difference (p<0.05) for the length of new shoots (L) and the mortality rate (Tm) (Table 5). The second-order cuttings showed the highest recovery rate (93%) compared to the others (90%), and a greater ability to elongate new shoots (14.9cm). On the other hand, these cuttings had a low mortality rate (7%). In contrast, those of order 3 showed the greatest ability to root elongation (13.8cm).
Table 5:Percentage recovery (%recovery); Number of buds (NB); Number of leaves/bud (NF/B); Number of roots (NR); Length of new shoots (L); Length of roots (LR) and mortality rate(Tm).
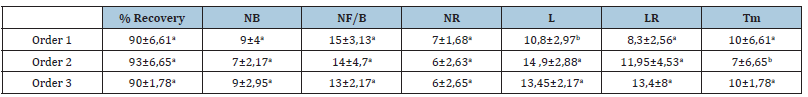
Note: For each parameter (row, column), the means followed by the same letter are not significantly different at the 5% threshold according to the Tukey test (n=5±standard deviation).
Correlation between the different morphological parameters under the effect order of woody cuttings
Table 6 showed that the variable number of roots has a positive and highly significant correlation (p<0.05)with root length(r=0.6691). However, it is negatively correlated with new shoot length (r=-0.0106) and number of leaves/bud (r=-0.2731). In addition, a negative correlation was recorded between the number of buds(NB) and the length of new shoots(L) (r=-0.2266).Whereas, the number of leaves/stem is negatively correlated with root length (r=-0.2982).
Table 6:Pearson’s correlation between the different morphological parameters of woody cuttings: Number of buds (NB), Number of leaves/bud (NF/B), Length of new shoots(L), Number of roots(NR), Length of roots(LR),(n=5).
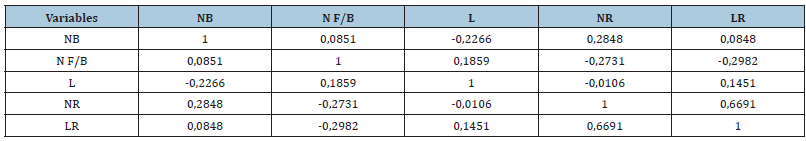
Note: For each parameter (row, column), the means followed by the same letter are not significantly different at the 5% threshold according to the Tukey test (n=5±standard deviation).
Propagation of the smooth caper by herbaceous cuttings
Analysis of variance of morphological parameters of herbaceous cuttings showed that there is a substrate and treatment effect on their growth (Table 7). In particular, for all substrates, there was no significant difference between control and cheated cuttings in the two treatmentsT1 (1000 ppm IBA) and T2 (2000ppm IBA). The maximum recovery is about 66.66%. On the other hand, the lowest rate was obtained in the T3 treatment (5000ppm AIB) with (53.33%); (61.66%) and (43.33%) for substrates 2; 3 and 4 respectively. However, among the treatments used, T1 showed an increase in the number of buds (3 buds) compared to the control in the S3 substrate (1/2 Sand+1/2 Peat).Interpretation of the results obtained for the number of leaves showed that substrate 1 is the most suitable for herbaceous cuttings treated with the two treatments 1000 and 5000ppm of IBA with 7 leaves. Treatment T2 significantly increased the number of roots (7 roots) at substrate 2. The best root length is obtained at substrate S2 for untreated cuttings (9.9cm).The best length of new shoots (9.3cm) and diameter (2.15mm) were measured in cuttings treated with T1.
Table 7:Mean recovery rates and morphological characters of herbaceous cuttings: Number of buds (NB), Number of leaves/bud(NF/B), Number of floral buds(NBF), Length of new shoots(L), Number of roots(NR), Length of roots(LR) and diameter(D), studied in 4 types of substrate(S1 (1/3 perlite + 1/3 sand +1/3peat), S2 (1/4 sand + 3/4peat), S3 (1/2 sand+ 1/2peat) and S4 (3/4 sand+ 1/4 peat) and 4 types of treatment (T0 : control; T1:1000ppm AIB; T2:2000ppm AIB and T3:5000ppm AIB)..
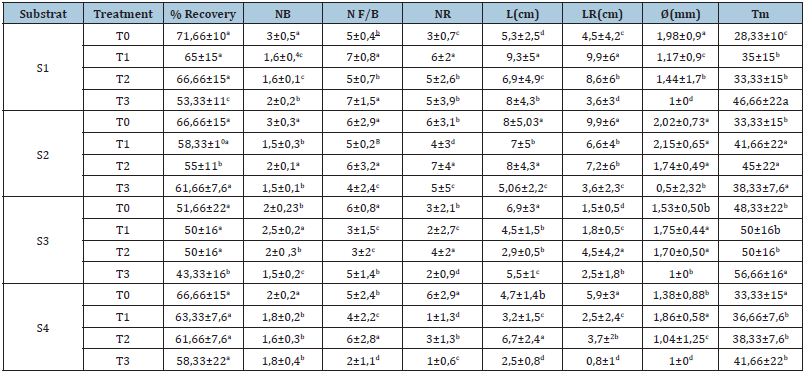
Note: For each parameter (row, column), the means followed by the same letter are not significantly different at the 5% threshold according to the Tukey test (n=5±standard deviation).
Correlation between morphological parameters of herbaceous cuttings
The Pearson correlation of the different morphological parameters of the herbaceous cuttings is recorded in Table 8. The length of new shoots showed a positive and highly significant correlation with the number of leaves/stem (r=0.7465), the number of roots (r=0.3912) and the length of roots (r=0.4358), while there was a negative correlation with the diameter at the crown (r=- 0.0077). In addition, a very weak correlation is reported between bud number(NB) and new shoot length (0.0409), NR(0.0867), LR(0.0228) and diameter (0.0958). While the number of buds is negatively correlated with the number of flower buds (r=-0.0409).
Table 8:Pearson correlation between different morphological parameters of herbaceous cuttings: Number of buds (NB), Number of leaves/stem (NF/B), Number of floral buds (NBF), Length of new shoots (L), Number of roots (NR), Length of roots (LR) and the diameter (D),(n=5).
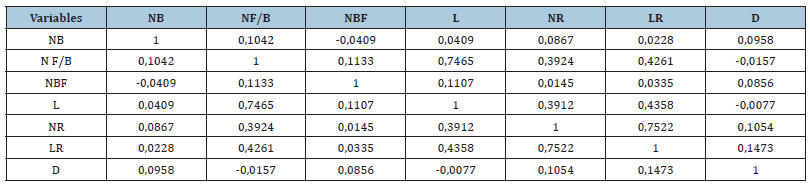
Note: For each parameter (row, column), the means followed by the same letter are not significantly different at the 5% threshold according to the Tukey test (n=5 ± standard deviation).
Discussion
Propagation of the smooth caper by woody cuttings
The present study showed the effect of substrate, hormonal treatment and branching order in relation to the mother plants on the success of woody and herbaceous cuttings of smooth caper. Considering all the results obtained, significant differences were observed. The results regarding the percentage of woody cuttings recovery are interesting and exceeded 80% for substrate 1 composed of perlite, sand and peat for which the D1 dose (2000ppm of AIB) gave the highest percentage 81.66%. The effect of AIB was clearly significant. However, these data are close to those of the work of Abidi [22], who found during propagation by cuttings of the smooth caper a percentage of recovery equal to 58.4% in a substrate of perlite + sand on a heated bottom (25-27 °C) with a treatment of 2000ppm of IBA. While Laribi et al. [23] showed that the percentage of recovery only exceeded 61.67% with 4000ppm AIB treatment in Rosa canina L. Concerning the number of buds, the most appropriate substrate is the one composed of (75% peat+25% sand) for both doses 2000 and 5000ppm. However, our results are superior to those obtained in woody cuttings propagation of Tunisian rose hip ecotype Laribi et al. [23] with (2.53±0.52) in the presence of 4000ppm IBA under sub-shelter prevailing conditions. In addition, the results obtained for the number of leaves showed that the most suitable substrates are S2 and S3. Treatment of cuttings with (5000ppm) showed the best result which is 3 times more as compared to the work of Laribi et al. [23] with a leaf count in the order of (6.53±0.52) recorded in rose hip cuttings treated with 4000ppm of IBA. While Ghazgazi [24] found that the best leaf count is around 15 leaves with 3000ppm IBA treatment in Rosa canina L. About the number of roots, it was noted that the most suitable substrate is S2 composed of 25% sand and 75% peat in woody cuttings soaked with 2000ppm IBA. Moreover, these results are very close to those reported by Khosh K [25] in Rosa damascene treated with 2000ppm with 10.19 roots per cutting. These data are inconsistent with those of Ghazgazi [24] where the number of roots reaches 16 roots with 3000ppm AIB treatment in Rosa canina L and those of Babaie et al. [26]where the number of roots reaches 9.20 roots with a treatment of 6000ppm AIB in Ficus benjamina L. As for the diameter of new shoots, the best result was obtained in the substrate composed of an equal portion of sand and peat with a treatment of 2000ppm of AIB. Regarding the morphological parameter of the length of new shoots, caper cuttings grown in substrate 1 (perlite+ sand+ peat) showed the best result. It should be noted that the control cuttings showed good length ability. These results are superior to those reported by Laribi et al. [23] in Rosa canina L. Treated with 4000ppm with length around 2.30±0.33cm. However, the results related to root length, the best value was obtained in substrate 2 based on 25% sand and 75% peat for cuttings treated with (5000ppm) of AIB. Note that our results were superior to those obtained by Khosh K [25] in Rosa damascena treated with 2000ppm AIB with(7.8cm) and by Laribi et al. [23] in rose hip treated with 4000ppm AIB with (2.12±0.27)cm. Regarding the branching order effect in relation to the mother plant of Capparis spinosa inermisthe percentage of recovery of woody cuttings exceeded 90% for all three branching orders. These results are superior to those reported by Diop et al. [27] in Jatropha curcas L, the percentage of recovery varied from 60 to 80% for order 1 branches and from 30 to 50% for order 3 branches. In addition, root length showed better growth in order 3 cuttings. These results coincide with those presented by Ait hammou et al. [28] where the best length is obtained at order 1(1.34cm) twigs in Argania spinosa L. ‘Skeels’.
Propagation of the smooth caper by herbaceous cuttings
Concerning vegetative propagation by herbaceous cuttings, interesting results have been obtained for different substrates. A recovery percentage of more than 66% was recorded for the substrate 1 composed of perlite, sand and peat in equal proportions, with a maximum of 71.66% for the control cuttings. On the other hand, these results are in contrast with those found by Laribi et al. [23] in Rosa canina L ‘where the 4000ppm dose of AIB showed the best results. However, according to Abidi [22] herbaceous cuttings of caper placed in heated perlite-based substrate and treated with AIB (2000ppm) gave the highest percentage of recovery 83.75%. On the subject of the number of buds, the most appropriate substrate is S3 (50% peat + 50% sand) in cuttings treated with 1000ppm AIB. These data are superior to those obtained in rose hips treated with 4000ppm IBA [22]. Interpretation of the results obtained for the number of leaves showed that substrate1 (Perlite+ Sand+ Turf) is the most suitable for herbaceous cuttings treated with the two doses 1000 and 5000ppm of IBA. In contrast, herbaceous cuttings in rose hip Laribi et al. [23] developed a lower leaf number (4.20±0.41) than our results with a 4000ppm hormone treatment. For the number of roots, it has been shown that the S2 substrate (25% sand and 75% peat) is the most suitable compared to the others. It should be noted that soaking the cuttings in IBA at 2000ppm significantly increased the number of roots. However, according to Dichel [29], herbaceous cuttings of the Sigoise olive variety placed in a substrate (perlite + sand) gave a lower number of roots (7 roots) than our results. About the length of new shoots, substrate 1(perlite+ sand+ peat), in the presence of T1 treatment (1000ppm), presented the best results which are higher than those reported by Laribi et al. [23] in Rosa canina L.(3.53±0.45) for cuttings treated by 4000ppm.
As for the root length parameter, the best substrate is sand (25%) and peat (75%) in the absence of hormonal treatment. Our results are greater than the average root length of olive cuttings grown (4cm) in a sand and perlite substrate [29]. Also, the root length is more improved in the inert caper, which was studied, compared to that of rose hip treated with 4000ppm of IBA reported in the work of Laribi et al. [23]. In fact, substrate S1 (1/3 perlite + 1/3 sand + 1/3 peat) showed the lowest mortality rate compared to the others. These results agree with those of Dichel [29] (25%) for herbaceous cuttings of Olive trees variety Sigoise.
Conclusion
This study was carried out to develop and know the techniques of vegetative multiplication of caper (Capparis spinosa inermis). Our results showed significant differences between the two types of cuttings (woody and herbaceous), the four types of substrate and the different treatments on the percentage of cuttings recovery, the mortality rate and some morphological parameters related to the quality of the root and aerial parts of the cuttings. Vegetative propagation using herbaceous or woody cuttings remains the most effective and recommended method. Indeed, the recovery percentages of woody and herbaceous cuttings were high (>66%) in a substrate composed of perlite, sand and peat. It seems that the herbaceous cuttings of the smooth caper are very sensitive to the chemicals presented by the growth hormone compared to the woody cuttings. Regarding the Indol-butyric acid (IBA) treatment, it presented a significant effect for the improvement of the propagation of Cappris spinosa inermis cuttings. While salicylic acid has no effect. Regarding the number of buds of woody cuttings, the most suitable substrate is the one composed of (75% peat + 25% sand) for both doses 2000 and 5000ppm with a number of 5 buds. Whereas, substrate 3 (50% sand + 50% peat) is the most suitable for herbaceous cuttings with an average of about 2.5 buds for the 1000ppm dose of IBA. In addition, the results obtained for the number of leaves showed that the most suitable substrates are S2 and S3 for woody cuttings. While, the results obtained for the herbaceous cuttings recorded the best number of leaves at the level of substrate 1(1/3 perlite, 1/3 sand and 1/3 peat). Regarding the length of new shoots of woody and herbaceous cuttings, the best results were obtained with a substrate composed of 1/3 perlite, 1/3 peat and 1/3 sand. In addition, the parameters of root number and root length were improved in substrate 2 (75% peat +25% sand) for both types of cuttings. The effect of branching order in relation to the mother plant, the percentage of recovery of woody cuttings of Capparis spinosa inermis showed non-significant results between orders but with a higher value (93%) for order 2 cuttings compared to the others. Since the number of flower buds that represent the production of capers, the number is very limited for both cutting techniques.
References
- Lakrimi M (1997) Le câprier-economic importance and technical conduct. Technology Transfer Bull in Agriculture. Direction of Plant Production MAEE Rabat, Marokko, p. 12.
- Chedraoui S, Abi RA, Beyrouthy ME, Chalak L, Ouaini N, et al. (2017) Capparis spinosa in a systematic review: A xerophilous species of multi values and promising potentialities for agro systems under the threat of global warming. Frontiers in Plant Science 8: 1845.
- Hall JC, Sytsma KJ, Iltis HH (2002) Phylogeny of capparaceae and brassicaceae based on chloroplast sequence data. American Journal of Botany 89(11): 1826-1842.
- Inocencio C, Rivera D, Maobon MC, Alcaraz F, Barrena JA (2006) A systematic revision of capparis section capparis (Capparaceae). Ann Missouri Boat Guard 93(1): 122-149.
- Benseghir BL, Seridi AR (2007) The caper bush, a scrub species for sustainable rural development in Algeria. Mediterranean [The caper, a scrub species for sustainable rural development in Algeria, Mediterranean]. Geographical Review of Mediterranean countries/Journal of Mediterranean geography 109: 101-105.
- Marouani A (2013) Caper cultivation in Tunisia in 70 questions and answers. Botany, Uses, Propagation, Cultivation, Harvest, Production, Conservation and Marketing, p. 78.
- Nabavi SF, Maggi F, Daglia M, Habtemariam S, Rastrelli L, et al. (2016) Pharmacological effects of capparis spinosa Phytother Res 30: 1733-1744.
- Fici S (2001) Intraspecific variation and evolutionary trends in Capparis spinosa (Capparaceae). Plant Systematics and Evolution 228(3): 123-141.
- Ghayour B, Mohammadi S, Sanchooli M, Pahlavanrav A (2013) The evaluation of effects of capparis spinosa on soil characteristics for management of rangeland a part of the environment. Intl J Agri Crop Sci 5(7): 785-788.
- Rhizopoulou S, Psaras GK (2003) Development and structure of drought tolerant leaves of the Mediterranean shrub Capparis spinosa Annals of Botany 92(3): 377-383.
- Özcan M (2005) Mineral composition of different parts of Capparis ovata Desf var. Canescens (Coss.) heywood growing wild in Turkey. J Med Food 8(3): 405-407.
- Moghaddasi MS, Kashani HH, Azarbad Z (2012) Capparis spinosa Propagation and medicinal uses. Life Science Journal 9(4): 684-686.
- Rivera D, Inocencio C, Obon C, Alcaraz F (2003) Review of food and medicinal uses of Capparis L. subgenus Capparis (Capparidaceae). Economic Botany 57(4): 515-534.
- Romeo V, Ziino M, Giuffrida D, Condurso C, Verzera A (2007) Flavor profile of Capers (Capparis spinosa) from the Eolian Archipelago by HS-SPME/GC-MS. Food Chem 101(3): 1272-1278.
- Infantino A, Tomassoli L, Peri E, Colazza S (2007) Viruses, fungi and insect pests affecting caper. The European Journal of Plant Science and Biotechnology 1(2): 170-179.
- Razouki A, Caper production in good shape. Lematin.
- Marouani A (1996) Contribution to the study of botanical aspects, modes of propagation and cultivation-techniques of caper in the Northwest of Tunisia. Final Report of the Caper Project in the North West of Tunisia, pp. 42-66.
- Ghorbel A, Fnayou BSA, Khouiledi S, Chibani F (2001) The caper Capparis spinosa L : Characterization and multiplication. Biological Models to Plant Improvement, pp. 157-172.
- Barbera G (1991) Agrimed research program: The caper bush (capparis spp) CEE Report EUR 13617 FR.
- Tsipouridis C, Thomidis T, Isaakidis A (2003) Rooting of peach hardwood and semi-hardwood cuttings. Australian Journal of Experimental Agriculture 43(11): 1363-1368.
- Boutherin D, Bron G (2013) Multiplication des plantes horticoles. Multiplication of horticultural plants. (3rdedn), Lavoisier France, p. 276.
- Abidi A (2014) Propagation by seed and cuttings and influence of Terracottem® on survival and vigor of rooted seedlings of inerme caper. Master's thesis. National Agronomic Institute of Tunisia, p. 126.
- Laribi B, Touil R, Kouki K, Bettaieb T (2013) Propagation by cuttings of a Tunisian eco-type of Rosehip (Rosa canina ). Nature & Technology (9): 55.
- Ghazgazi H (2012) Towards an exploitation of the Rosa genus in the Kroumirie region (North-western Tunisia): Rosa canina and Rosa sempervirens L. Doctoral thesis, p. 163.
- Khosh KM, Tafazoli E (1979) Effect of acid or base pretreatment on auxin response of damask rose cuttings. Scientia Horticulturae 10(4) : 395-399.
- Babaie H, Zarei H, Hemmati K (2014) Propagation of Ficus benjamina var. starlight by stenting technique under different concentration of IBA in various time of taking cutting. Journal of Ornamental Plants 4(2): 75-79.
- Diop B, Samba SAN, Akpo LE (2012) Morphological characteristics and growth of seedlings of Jatropha curcas International Journal of Biological and Chemical Sciences 6(2) : 677-691.
- Ait HR, Daoud S, Harrouni MC (2018) Effect of age, clone heads, ramet position and AIB treatment on the rooting of argan tree (Argania spinosa L. Skeels) cuttings. p. 8.
- Dichel A (2017) Study of the influence of the sub-strate on the rhizogenesis of herbaceous cuttings of olive trees variety sigoise propagated in greenhouse nebulization ]. Master's thesis, Faculty of natural and life sciences and earth sciences of the universe, p. 87.
© 2023 Benaoun Hanine. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






